Ovarian cancer screening methods are in urgent need of improved accuracy. When detected early, at stages 1 or 2, the disease has a good prognosis: Surgery and chemotherapy can eliminate the disease for 70 to 90 percent of patients. However, for those diagnosed later, this rate drops dramatically—to 20 percent or fewer, according to a study published in Ultrasound in Obstetrics and Gynecology. Fortunately, screening methods such as biomarkers and ultrasound protocols are constantly advancing.
How Ovarian Cancer Is Diagnosed
Clinicians often identify adnexal masses during routine annual examinations, or incidentally during imaging for pelvic symptoms. They typically use ultrasound imaging and serum biomarker testing to accurately identify which adnexal masses are benign and which may be cancerous.
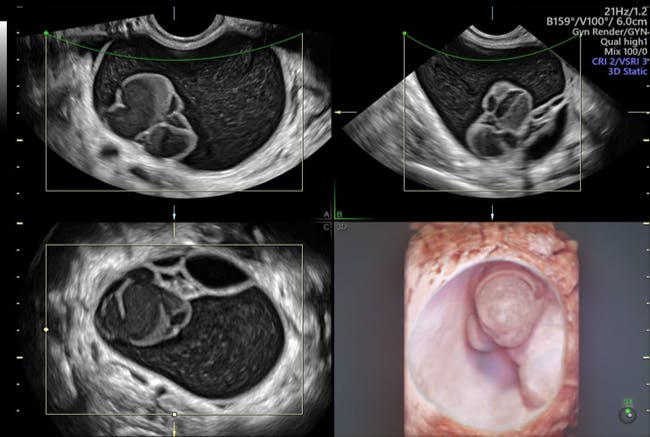
3D ultrasound improves diagnostic accuracy when assessing potential ovarian cancer. Color Doppler evaluation can show the presence of vascularity—which may indicate metastatic cancer—and the central localization of vessels in a tumor. The International Ovarian Tumour Analysis (IOTA) group's Simple Rules play a critical role in helping clinicians differentiate between benign and malignant adnexal masses on Doppler ultrasound. The IOTA rules make it easy to classify masses by solidity, contents, and other features, and are a built-in feature on Voluson™ ultrasound equipment.
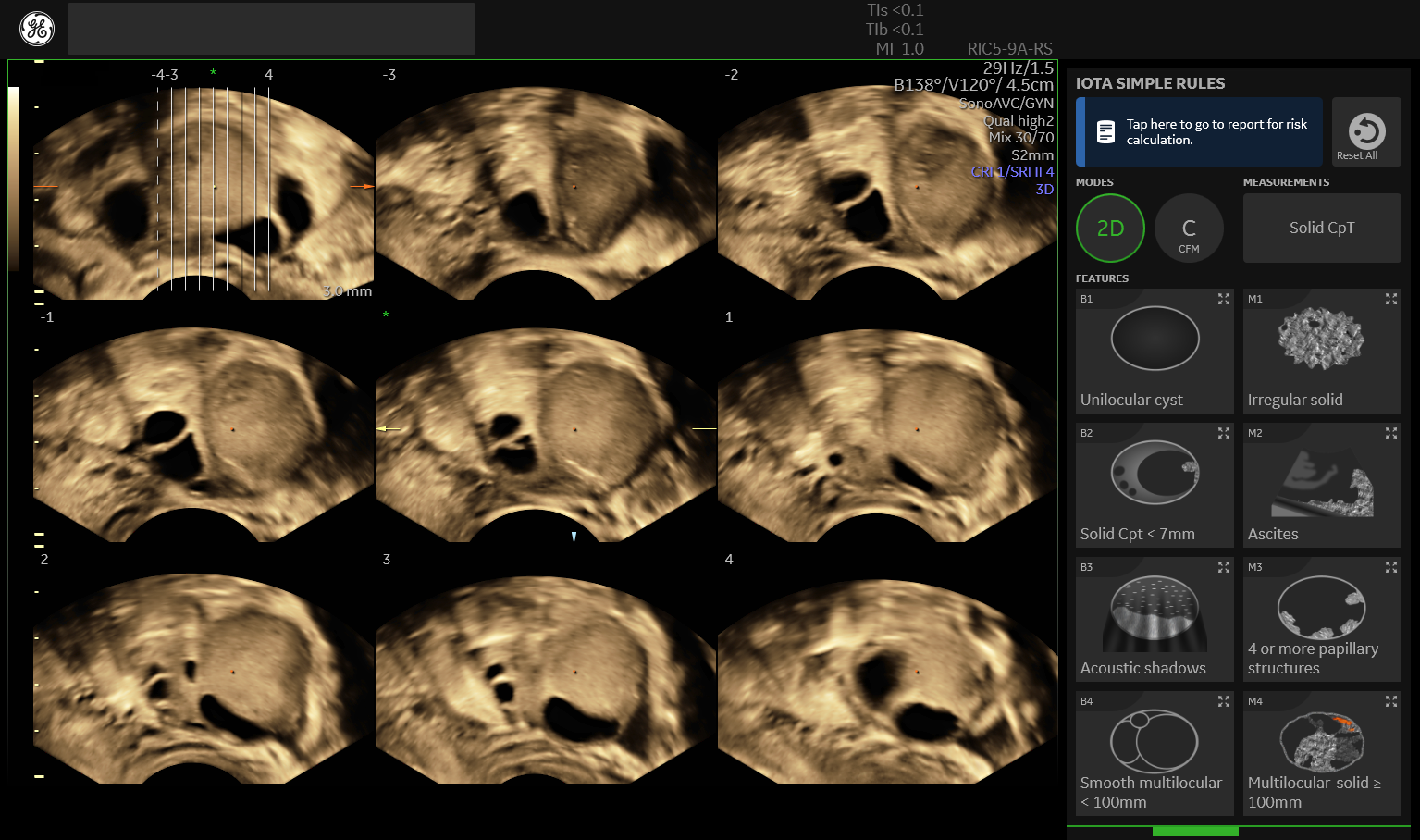

IOTA guidelines imbedded in Voluson™ products to support imagers
The Ovarian-Adnexal Reporting and Data System (O-RADS) is an additional set of guidelines that aims to streamline the terminology and diagnostic criteria used to classify adnexal masses. The creators of O-RADS intended these guidelines to aid accurate communication between sonographers and referring clinicians about the details of adnexal masses—a critical step if a provider wants to move a patient from ultrasound examination to biomarker testing.
Current Biomarker Protocols
The serum biomarker CA125 has long been a primary evaluation tool for assessing women with adnexal or pelvic masses, according to a review published in Contemporary OB/GYN. This protein is expressed in the mesothelial lining of the pleura and peritoneum as well as in other epithelial tissues. According to the review, CA125 is not usually expressed by the epithelium of noncancerous ovaries, but 90 percent of women with metastatic ovarian cancer will show elevated levels of CA125 in serum.
CA125 has low sensitivity and specificity, however. Per the review in Contemporary OB/GYN, preoperative CA125 levels are normal in around 50 percent of women with stage 1 ovarian cancer and can be elevated during events such as ovulation and menstruation, as well as in women with colon and pancreatic cancers.
Due to the limitations of CA125, researchers have continued the hunt for additional biomarkers, including combinations of serum markers. Some have found that human epididymis protein 4 can be used in concert with CA125 in the Risk of Ovarian Malignancy Algorithm (ROMA) to assess a patient's risk, according to a study published in Obstetrics and Gynecology. After an adnexal mass is initially discovered using imaging such as ultrasound, ROMA categorizes patients as high or low risk based on a predictive index that includes menopausal status, CA125 and HE4 assay results. Validation studies led to the Food and Drug Administration's approval of ROMA in 2011.

IOTA Risk Assessment Models
Current Biomarker Protocols
Researchers continue to search for biomarkers that can improve the early detection of ovarian cancer. A recent study published in Cancer Prevention Research measured 92 cancer-related proteins, 40 of which have the potential to serve as biomarkers for ovarian cancer. Another recent study published in Cancers used novel filtering procedures to find "thousands of MS (mass spectrometry) glycopeptide peaks that showed statistically significant differences between ovarian cancer patients and control patients."
The researchers used liquid chromatography with mass spectrometry, a new approach to discovering biomarkers. They looked at not just sugar chain alterations, but combinations of both protein and glycan changes. The study used a small sample size, so additional validation and development of the method are needed. Nonetheless, the results suggest that comprehensive serum glycopeptide spectrum analysis could be a very valuable tool for early detection of ovarian cancer, and potentially other cancers as well.
Biomarker protocols may one day form the backbone of ovarian cancer screening, but for now, clinicians should rely on a more readily available tool: ultrasound. ORADS and IOTA are critical protocols for helping detect a malignant adnexal mass as early as possible and leading your patient to the right treatment.