When a patient has a low antral follicle count (AFC), low anti-Müllerian hormone (AMH) level, or high follicle-stimulating hormone (FSH) level, they in turn have a lower chance of success with fertility treatments. For patients who have just been told they need chemotherapy or radiation, time is of the essence if they want to have biological children in the future.
The IVF stimulation protocol known as the luteal phase protocol, with or without dual stimulation (DuoStim), may offer these patients hope. Reproductive endocrinologists should understand DuoStim, previously called the Shanghai Protocol, and luteal phase protocols to better help fertility preservation and patients with diminished ovarian reserve (DOR).
Overview of the IVF Dual Stimulation Protocol
Researchers previously believed that follicular recruitment only happened once during the menstrual cycle—at the beginning of the follicular phase. However, research published in Human Reproduction Update found that a patient's ovaries naturally recruit follicles in several rounds, typically two to three during each menstrual cycle.
Regardless of when recruitment takes place, hormonal cues from the brain allow only one follicle, containing one oocyte, to be chosen. Other follicles never mature, and the oocytes within them dissolve.
During IVF, on the other hand, ovarian stimulation medications are used to mature some of the oocytes that the body would otherwise discard, allowing reproductive endocrinologists to retrieve them with the hope that they fertilize and develop into healthy blastocysts.
Unlike the traditional IVF approach, which involves one cycle of ovarian stimulation and one oocyte retrieval during one menstrual cycle, dual stimulation involves two cycles of stimulation and two oocyte retrievals during the same menstrual cycle.
This process may follow one of two sets of steps. The first is a follicular phase stimulation, followed by an oocyte retrieval and then restarting stimulation in the luteal phase. The second begins with luteal phase stimulation, followed by an oocyte retrieval and restarting stimulation in the new luteal phase initiated by the retrieval.
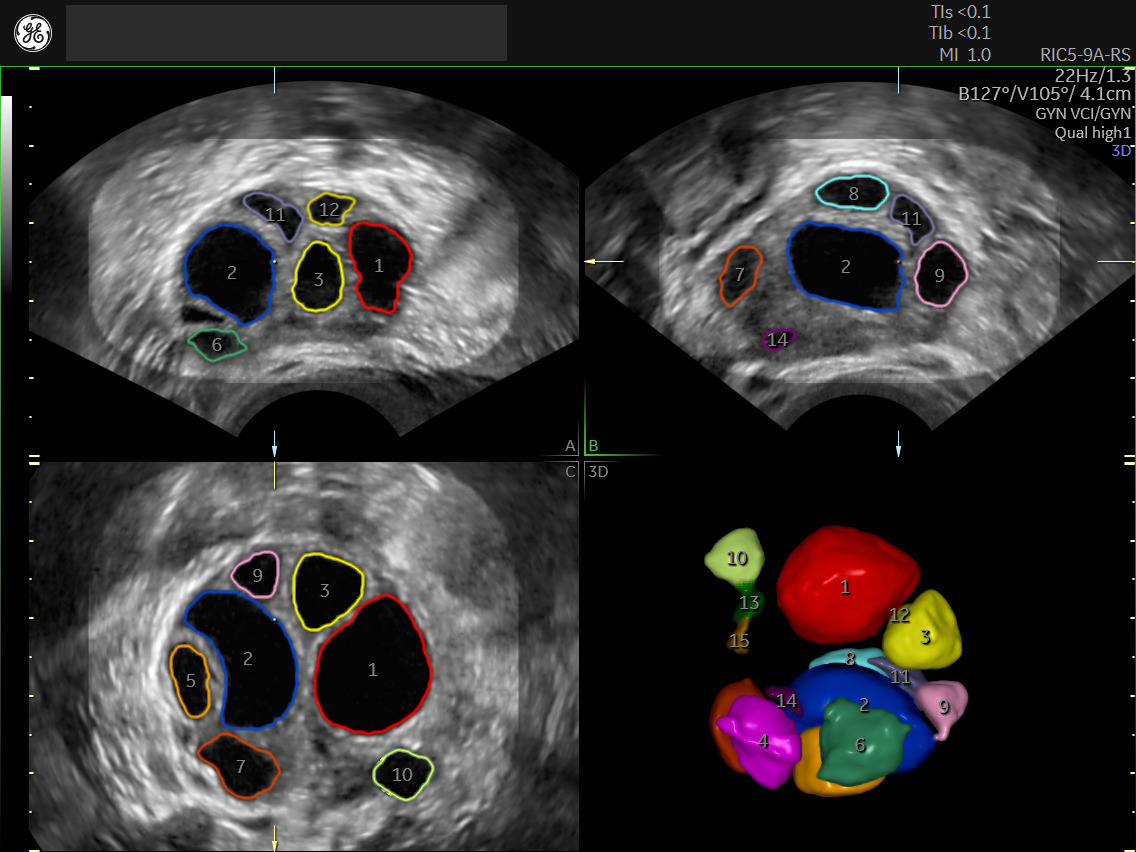
SonoAVC™ follicle enhances the efficiency and reproducibility of follicular volume measurements to effectively monitor stimulation and determine the optimal time point for oocyte retrieval.
Overview of the Luteal Phase Protocol
Most IVF patients follow a follicular phase protocol. As the name implies, this ovarian stimulation takes place during the follicular phase. A luteal phase protocol, likewise, entails stimulation during the luteal phase. Similar to a follicular phase protocol, patients go to their fertility clinic for a baseline ultrasound—which case studies show are integral to the IVF process—and blood work prior to beginning stimulation.
However, this holds one major difference: Rather than the baseline appointment taking place on the second or third day of the patient's menstrual cycle, as it would during a follicular phase protocol, the baseline is established between two and seven days after ovulation.
In terms of ovarian stimulation, clomiphene citrate (Clomid) or letrozole with or without low-dose exogenous gonadotropins (FSH, LH or a combination of the two) marks one option to minimize the risk of hyperstimulation. Another possibility is conventional ovarian stimulation—moderate to high exogenous FSH with or without luteinizing hormone (LH) and a low dose of human chorionic gonadotropin (hCG).
To avoid a premature LH surge and ovulation, physicians may use a gonadotropin-releasing hormone antagonist (GnRH-ant), such as Cetrotide or Ganirelix, or exogenous progestins. Typically, the use of GnRH-ant or progestin begins when the lead follicle measures between 12 to 14 mm and discontinues when ovulation is triggered.
The same Human Reproduction Update study that examined natural recruitment in the ovaries found that a dual trigger of HCG and a gonadotropin-releasing hormone agonist (GnRH-a) such as Lupron best encourages last-minute oocyte maturation. This procedure resulted in more oocytes being retrieved, fertilized and developed into high-quality embryos, according to the findings.
Defining Poor Prognosis
Accurate antral follicle counts and measurements have a vital role in every ovarian stimulation cycle, but especially so for patients with a poor prognosis.
So-called poor responders are often defined using the Bologna criteria, a consensus published in Human Reproduction by the European Society for Human Reproduction and Embryology (ESHRE) in 2011. The criteria include:
- Maternal age greater than 40
- A result of 3 embryos or fewer during previous ovarian stimulation
- An ovarian reserve of fewer than 5-7 follicles in antral follicle count, or less than 0.5-1.1 ng/ml AMH
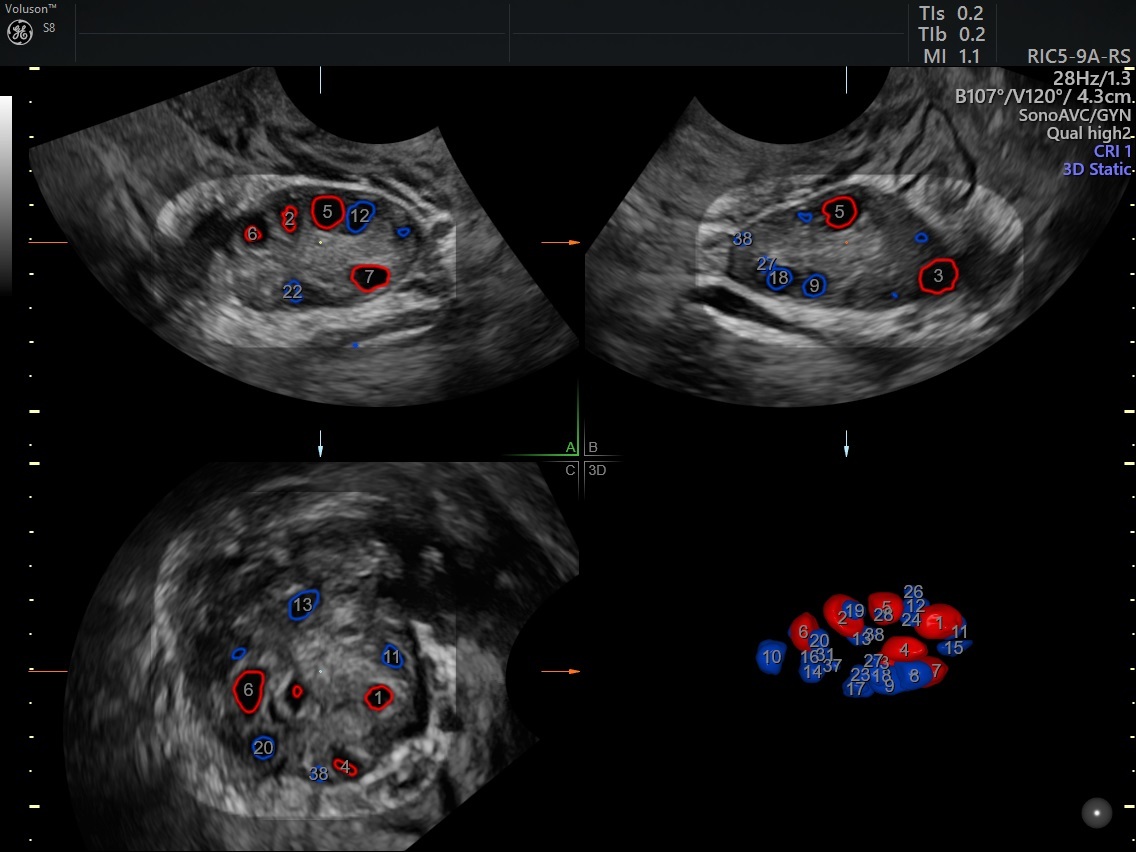
SonoAVC™antral automates ovarian reserve assessment identifying and counting antral follicles.
Evaluating the Efficacy of the Dual Stimulation Protocol
The purpose of DuoStim is to collect as many oocytes as possible in the shortest amount of time. This can benefit both patients who require fertility preservation and those who are poor responders.
A January 2020 study published in Fertility and Sterility evaluated the efficacy of dual stimulation for patients with a poor prognosis, identified using the Bologna criteria. Both the first stimulation cycle, which was performed during the follicular phase, and the second stimulation cycle, which was performed during the luteal phase, resulted in similar fertilization and blastocyst rates. However, the second stimulation increased the number of oocytes retrieved, the likelihood of getting at least one euploid blastocyst and the live birth rate.
A study published in Reproductive Biology and Endocrinology also reports several differences in patient response during the luteal phase portion of an IVF dual stimulation protocol. The hormone levels monitored—estrogen (E2), progesterone (P4) and luteinizing hormone (LH)—were found to be higher, and the total number of follicles was significantly higher. Perhaps most importantly, the pregnancy rate was higher for embryos that resulted from luteal phase stimulation. Miscarriage rates were unaffected.
Further, the Human Reproduction Update study found that dual stimulation cycles are less likely to be cancelled. A Journal of Obstetrics and Gynaecology Research article reports that embryo transfers following an IVF dual stimulation protocol also have a lower chance of being cancelled, presumably because these patients are more likely to have a euploid embryo available for transfer.
Despite findings demonstrating that IVF dual stimulation cycles and luteal phase protocols result in better patient outcomes and may even be more cost effective, the process still has limitations. All embryos must be cryopreserved for transfer at a later date, which some patients may not prefer. Reproductive endocrinologists should inform patients about these options so they can make the best decision for their individual situation.