Published online in Wiley Online Library (wileyonlinelibrary.com). DOI: 10.1002/uog.19072
ISUOG Practice Guidelines: intrapartum ultrasound
Clinical Standards CommitteeThe International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) is a scientific organization that encourages sound clinical practice and high-quality teaching and research related to diagnostic imaging in women’s healthcare. The ISUOG Clinical Standards Committee (CSC) has a remit to develop Practice Guidelines and Consensus Statements as educational recommendations that provide healthcare practitioners with a consensus-based approach, from experts, for diagnostic imaging. They are intended to reflect what is considered by ISUOG to be the best practice at the time at which they were issued. Although ISUOG has made every effort to ensure that Guidelines are accurate when issued, neither the Society nor any of its employees or members accepts any liability for the consequences of any inaccurate or misleading data, opinions or statements issued by the CSC. The ISUOG CSC documents are not intended to establish a legal standard of care, because interpretation of the evidence that underpins the Guidelines may be influenced by individual circumstances, local protocol and available resources. Approved Guidelines can be distributed freely with the permission of ISUOG ([email protected]).
PURPOSE AND SCOPE
The purpose of these Guidelines is to review the published techniques of ultrasound in labor and their practical applications, to summarize the level of evidence regarding the use of ultrasound in labor and to provide guidance to practitioners on when ultrasound in labor is clinically indicated and how the sonographic findings may affect labor management. We do not imply or suggest that ultrasound in labor is a necessary standard of care.
BACKGROUND AND INTRODUCTION
Traditionally, the assessment and management of a woman in labor is based upon clinical findings1–7. The diagnosis of arrest of labor and decisions regarding the timing or type of intervention rely mostly on digital evaluation of cervical dilatation and fetal head station and position8–17. However, clinical examination of head station and position is inaccurate and subjective18–25, especially when caput succedaneum impairs palpation of the sutures and fontanels. The use of ultrasound has been proposed to aid in the management of labor. Several studies have demonstrated that ultrasound examination is more accurate and reproducible than clinical examination in the diagnosis of fetal head position and station19–33 and in the prediction of arrest of labor34–42. Ultrasound examination can, to some extent, distinguish those women destined for spontaneous vaginal delivery and those destined for operative delivery43–47. Furthermore, there is growing evidence that ultrasound in labor may predict the outcome of instrumental vaginal delivery44–48. Ultrasound in labor can be performed using a transabdominal approach, mainly to determine head and spine position49, or a transperineal approach, for assessment of head station and position at low stations. Several quantitative sonographic parameters have been proposed to assess head station30–32,34,35,40,42,43,50,51. Currently, there is no consensus regarding when in labor ultrasound should be performed, which parameter(s) should be obtained and how the sonographic findings should be integrated into clinical practice in order to improve management of the patient.
IDENTIFICATION AND ASSESSMENT OF EVIDENCE
The Cochrane Library and Cochrane Register of Controlled Trials were searched for relevant randomized controlled trials, systematic reviews and meta-analyses. A search of Medline from 1966 to 2017 was also carried out. The date of the last search was 30 September 2017. In addition, relevant conference proceedings and abstracts were searched. Searches used the relevant MeSH terms, including all subheadings. This was combined with a keyword search, including: ‘labor ultrasound’, ‘transperineal ultrasound’, ‘fetal head station’, ‘fetal occiput position’ and ‘instrumental vaginal delivery’. When possible, recommendations in these Guidelines are based on, and explicitly linked to, supporting evidence. Details of the grades of recommendation and levels of evidence used in these Guidelines are given in Appendix 1.
GUIDELINES
Aims of ultrasound in the labor ward
These Guidelines address exclusively the use of ultrasound in labor to determine fetal head station, position and attitude. All other applications of ultrasound in the labor ward, such as assessment of cervical length or dilatation and fetal Doppler studies, are not covered. For the time being, ultrasound should be used as an adjunctive method and not as a substitute for clinically indicated digital vaginal examination.
Assessment of fetal head position
Precise knowledge of fetal occiput position in labor is of paramount importance.- Persistent occiput-posterior position is associated with higher risk of operative delivery52 and maternal and perinatal morbidity53,54.
- Correct determination of head position is crucial before instrumental delivery. An error in evaluation of head position may result in inappropriate vacuum or forceps placement, increasing the potential for fetal injury and the failure rate of the procedure55–58. Failed instrumental delivery followed by Cesarean section is associated with an increased decision-to-delivery interval59 and an increased risk of maternal60,61 and fetal62–65 trauma.
Traditionally, clinicians determine fetal head position
by palpating the sagittal suture and the anterior and
posterior fontanels. Several studies have evaluated the
accuracy of clinical diagnosis of fetal head position,
using ultrasound19–28 or position-tracking technology
systems66 as reference; digital palpation was found to be
subjective. Studies show consistently that digital examination
to determine head position is inaccurate, with
a rate of error ranging from 20% to 70%, when
considering ultrasound as the standard19 (LEVEL OF
EVIDENCE: 1–).
Clinical evaluation by palpation tends to be even less
accurate in cases of abnormal head position, such as
occiput posterior or transverse, when medical intervention
is more likely to be needed19,20,22,23 (LEVEL OF
EVIDENCE: 2++).
This inaccuracy may be exaggerated by the presence
of caput succedaneum and asynclitism, both of
which are frequently associated with obstructed labor.
Several studies have failed to demonstrate a significant
difference in accuracy between experienced and
inexperienced obstetricians19,21,22, although this finding
has been questioned by others20 (LEVEL OF
EVIDENCE: 2+).
Various studies have demonstrated the superiority of
ultrasound alone or in combination with digital examination
in the precise determination of fetal head rotation
as compared with traditional digital examination
alone19–28,66 (LEVEL OF EVIDENCE: 1–).
Assessment of fetal head position
The fetal head station is the level of the fetal head in the
birth canal relative to the plane of the maternal ischial
spines (non-cephalic presentation is not considered in
these Guidelines). The term ‘head engagement’ is used
when the widest part of the head passes into the pelvic
inlet or two-fifths or less of the fetal head is palpable
abdominally, corresponding to descent of the biparietal
plane of the fetal head to a level below that of the pelvic
inlet67. On digital vaginal examination, the fetal head is
considered engaged when the leading part of the skull has
reached the imaginary line or plane between the maternal
ischial spines. This head station is referred to as station 0.
Higher or lower head stations are expressed in centimeters
above (negative) or below (positive) this reference plane,
respectively.
The subjectivity of transvaginal digital assessment of
fetal head station was demonstrated by Dupuis et al.18
(LEVEL OF EVIDENCE: 2+). Using a birth simulator
equipped with a sensor, they placed a fetal head
mannequin at defined stations according to the American
College of Obstetricians and Gynecologists, and
a group of examiners of various levels of experience
used palpation to classify the fetal head station as high,
mid-pelvis, low or outlet. The mean ‘category’ error
was 30% for residents and 34% for obstetricians. More
importantly, the incorrect diagnosis of a mid-pelvic station
rather than a true high-pelvic station accounted for
the majority of errors (88% and 67% by residents and
obstetricians, respectively). In clinical practice, such misclassification
may impact adversely on the management
of labor.
Ultrasound examination documents objectively and
precisely the fetal head station in the birth
canal29–33,35,47,68 (LEVEL OF EVIDENCE: 2+).
A series of sonographic parameters have been suggested
to describe the fetal head station; these have
been demonstrated to have high intra- and interobserver
agreement69–71 (LEVEL OF EVIDENCE: 2+).
Assessment of fetal head descent (progression)
Some observational studies36,37,39,72,73 have suggested that repeat ultrasound examinations to assess the change of head station over time (‘progression’) performs better than does digital examination in documenting fetal head descent and in demonstrating slow labor or lack of progress in both the first and second stages (LEVEL OF EVIDENCE: 2+).
Assessment of fetal head attitude
The fetal head attitude is the relationship of the fetal head to spine. Ultrasound has proved helpful in visual assessment of fetal head attitude74,75 (LEVEL OF EVIDENCE: 2–) and in the objective diagnosis of fetal head malpresentation in labor76–80 (LEVEL OF EVIDENCE: 3).
Technique
Ultrasound assessment in labor may be performed using a transabdominal or transperineal approach, depending on the parameter that is the aim of the examination (mainly position and station) and on the clinical indication. A two-dimensional ultrasound machine equipped with a convex probe, such as that used for transabdominal fetal ultrasound for biometry and assessment of anatomy, is used. Suggested requirements of equipment for use in the labor ward are that it is quick to start up, and has batteries with a long life and that are quick to recharge. A wide-sector, low-frequency (<4MHz) insonation is best suited to ultrasound in labor.
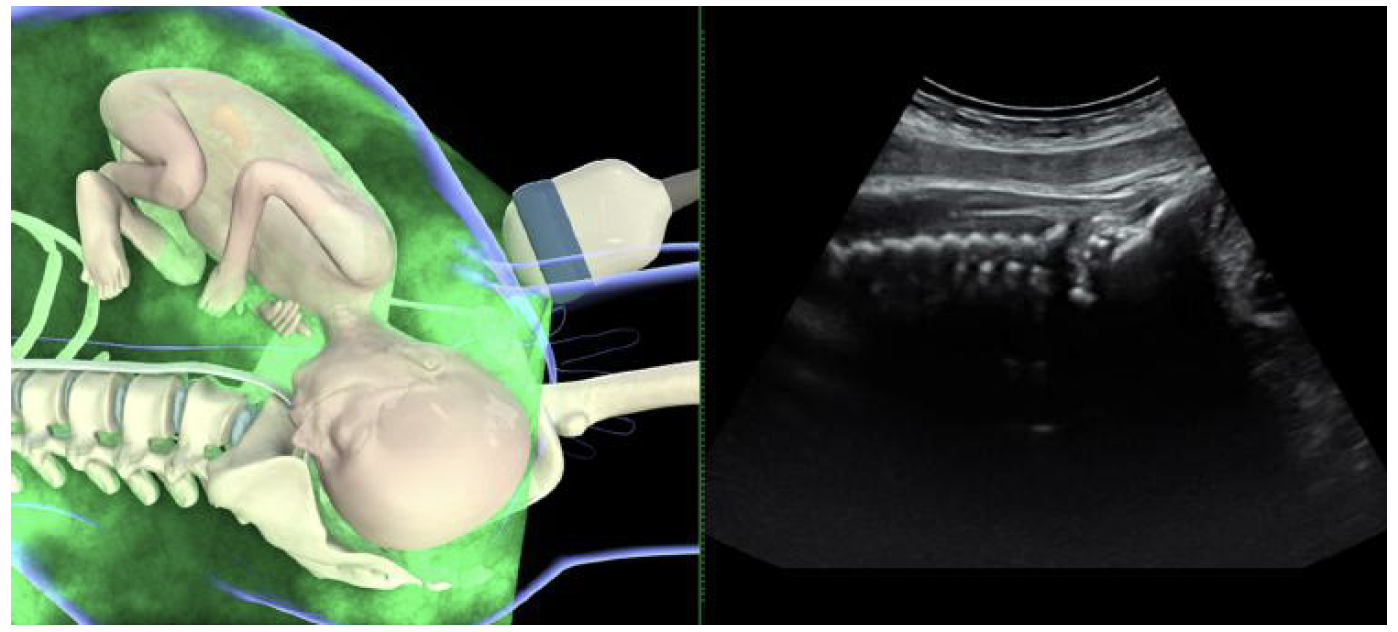
Assessment of fetal head position
Sonographic assessment of fetal head position is best performed
by transabdominal imaging in axial and sagittal
planes81. Placing the ultrasound probe transversely on
the maternal abdomen, an axial view of the fetal trunk is
obtained at the level of the fetal upper abdomen or chest.
The position of the fetal spine may then be determined.
The ultrasound transducer is then moved downwards until
it reaches the maternal suprapubic region, visualizing the
fetal head. The landmarks depicting fetal occiput position
are the two fetal orbits for occiput posterior, the midline
cerebral echo for occiput transverse, and the occiput itself
and the cervical spine for occiput-anterior position81
(Figures 1 and 2). The choroid plexus, which diverges
towards the occiput, can be helpful in determining fetal
head position47.
The midline structures in the fetal head may be difficult
to visualize on transabdominal imaging at low fetal head
stations. Combining a transabdominal with a transperineal
ultrasound approach may be recommended in these
cases for precise determination of position.
Position can be described by depicting a circle, like
a clock (Figure 3): positions ≥02.30 h and≤03.30 h
should be recorded as left occiput transverse (LOT);
positions ≥08.30 h and≤09.30 h as right occiput transverse
(ROT); positions >03.30 h and<08.30 h should
be recorded as occiput posterior; and positions >09.30 h
and<02.30 h as occiput anterior25.
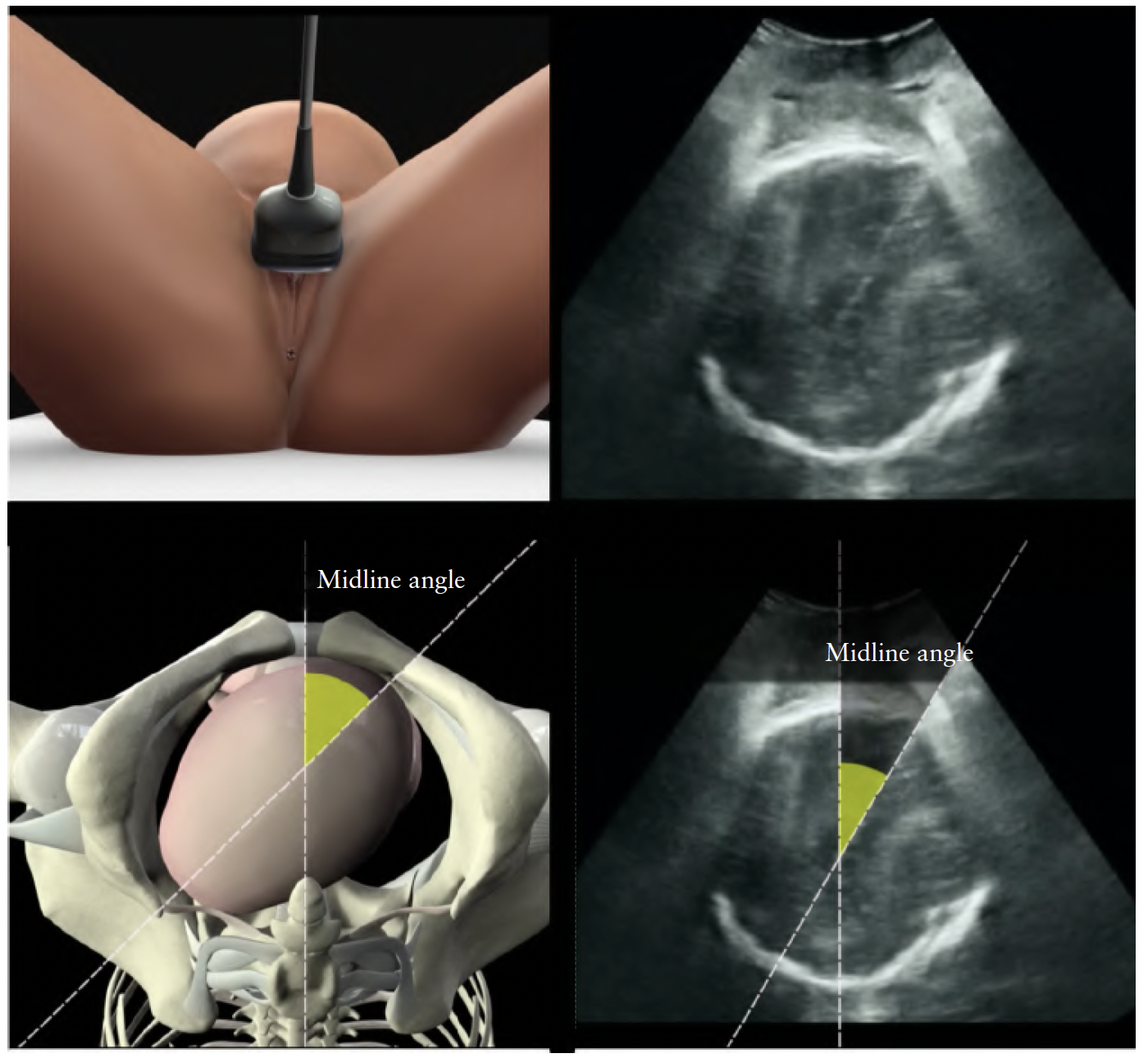
Assessment of fetal head station
Sonographic assessment of fetal head station is best performed by transperineal ultrasound in the midsagittal or axial plane. The probe is placed between the two labia
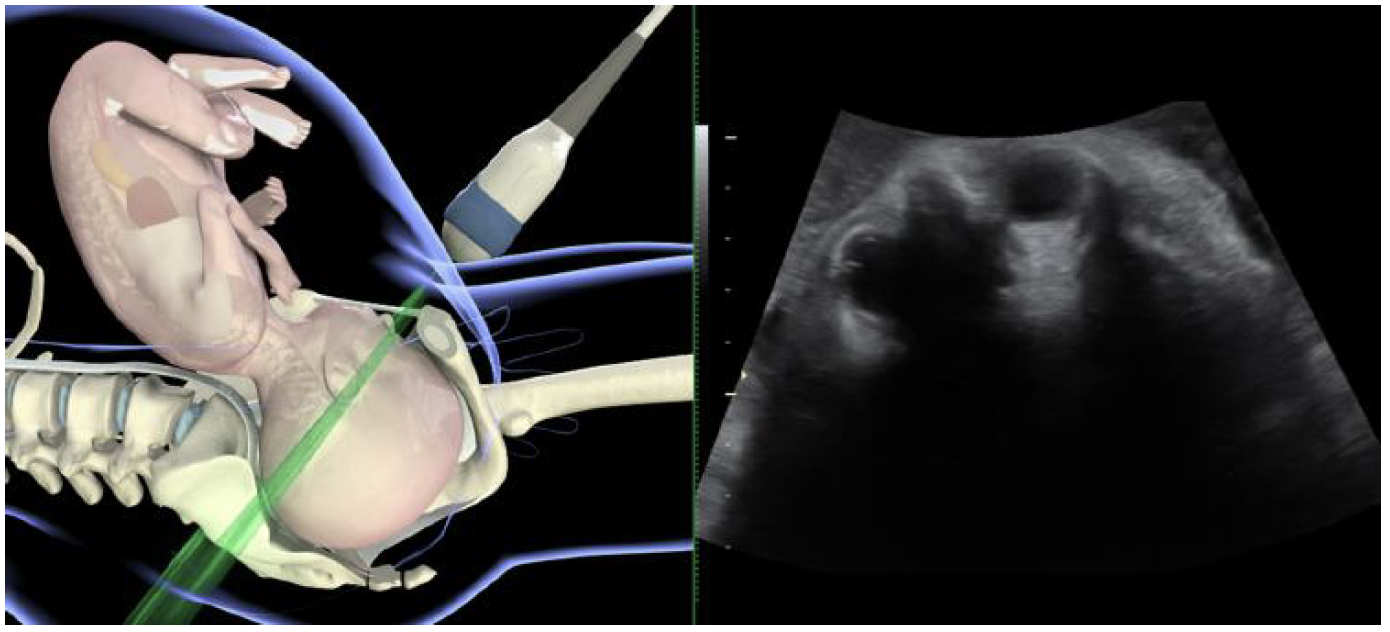
majora or more caudally, at the level of the fourchette, with the woman in a semirecumbent position, with legs flexed at the hips and knees at 45◦ and 90◦ degrees, respectively. It is essential that her bladder is empty. In the midsagittal plane, the following anatomical landmarks are clearly depicted:
- pubic symphysis joint, as an oblong, irregular, echogenic structure; ideally displayed in a horizontal position;
- fetal skull, with anterior and posterior tabula.
The traditional reference plane of vaginal palpation,
the level of the ischial spines, cannot be seen in this view.
However, there is a fixed anatomical relationship between
the lower end of the pubic symphysis and the interischial
plane: the ‘infrapubic line’ is an imaginary line originating
from the caudal end of the symphysis pubis, perpendicular
to its long axis, extending to the dorsal part of the birth
canal. In three-dimensional reconstructions of computed
tomographic data from a normal female bony pelvis, the
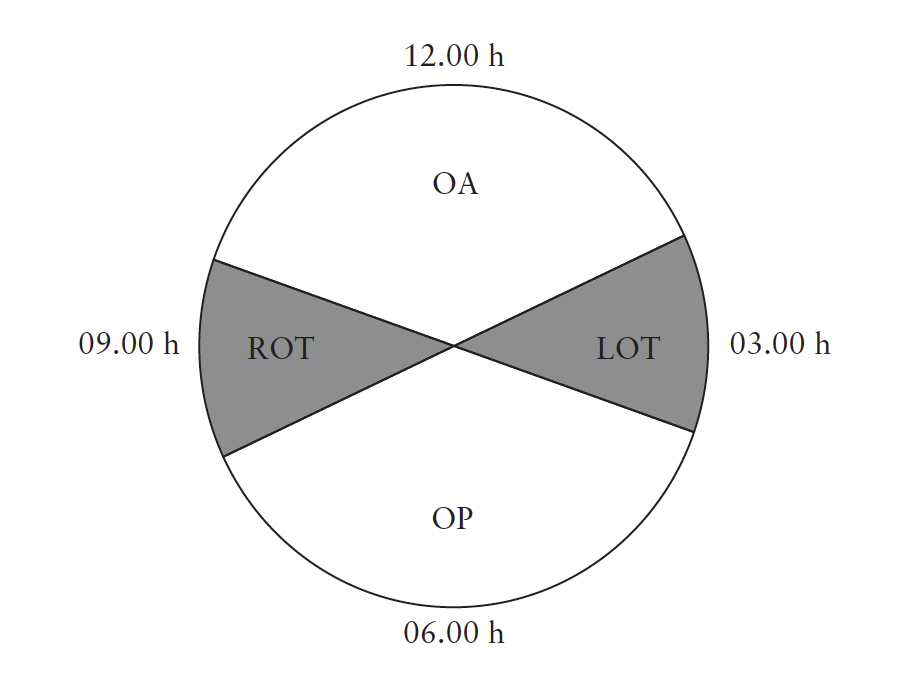 Figure 3 Classification of fetal occiput position based on positions
of hour hand on a clock face: positions ≥02.30 h and≤03.30 h
should be recorded as left occiput transverse (LOT) and positions
≥08.30 h and≤09.30 h as right occiput transverse (ROT).
Positions >03.30 h and<08.30 h are occiput posterior (OP) and
positions >09.30 h and<02.30 h are occiput anterior (OA)92,93.
Figure 3 Classification of fetal occiput position based on positions
of hour hand on a clock face: positions ≥02.30 h and≤03.30 h
should be recorded as left occiput transverse (LOT) and positions
≥08.30 h and≤09.30 h as right occiput transverse (ROT).
Positions >03.30 h and<08.30 h are occiput posterior (OP) and
positions >09.30 h and<02.30 h are occiput anterior (OA)92,93.
infrapubic line has been shown to be 3 cm above the plane
of the ischial spines42,82–84.
On transperineal imaging in the midsagittal plane, several
parameters have been proposed that use the pubic
symphysis as landmark and reference point for quantitative
measurements. Three indicate head station directly:
the angle of progression (AoP), also called the ‘angle of
descent’40,43; the progression distance (PD)30; and the
transperineal ultrasound head station41. Others indicate
it indirectly: the head–symphysis distance (HSD) is an
indirect parameter that changes with descent51; and the
head direction indicates the direction of the longest recognizable
axis of the fetal head with respect to the long
axis of the pubic symphysis42.
With simple clockwise rotation of the transducer by
90◦, an axial plane is obtained, in which two additional
parameters can be evaluated and measured: the
head–perineum distance (HPD)34, as a marker of head
station; and the midline angle (MLA)31, which assesses
rotation of the head.
Angle of progression (AoP)/angle of descent. The AoP is
the angle between the long axis of the pubic bone and a
line from the lowest edge of the pubis drawn tangential
to the deepest bony part of the fetal skull (Figure 4). It
was first described in 200940,43 and has been found to be
an accurate and reproducible parameter for assessment of
fetal head descent40,41,69,70 (LEVEL OF EVIDENCE: 2+).
D uckelmann et al.72 demonstrated that measurement of
AoP can be learned easily, regardless of the clinician’s
level of ultrasound experience (LEVEL OF EVIDENCE:
2+). In their investigation of several different parameters,
Tutschek et al.41 compared AoP and transperineal
ultrasound head station, finding that fetal [head station 0
corresponds to an AoP of 116◦ (Table 1).
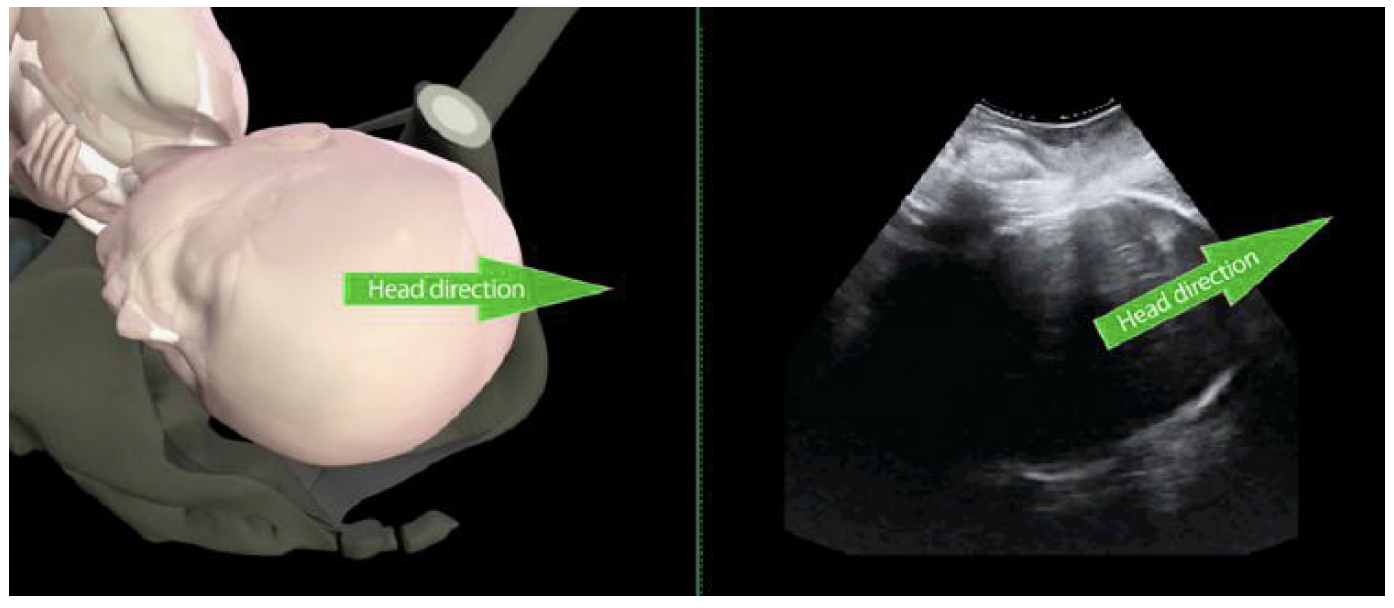
Fetal head direction. Head direction, an indirect marker
of head station, was first described by Henrich et al.42, as
the angle between the longest recognizable axis of the fetal
head and the long axis of the pubic symphysis, measured
in a midsagittal transperineal view (Figure 5). It was
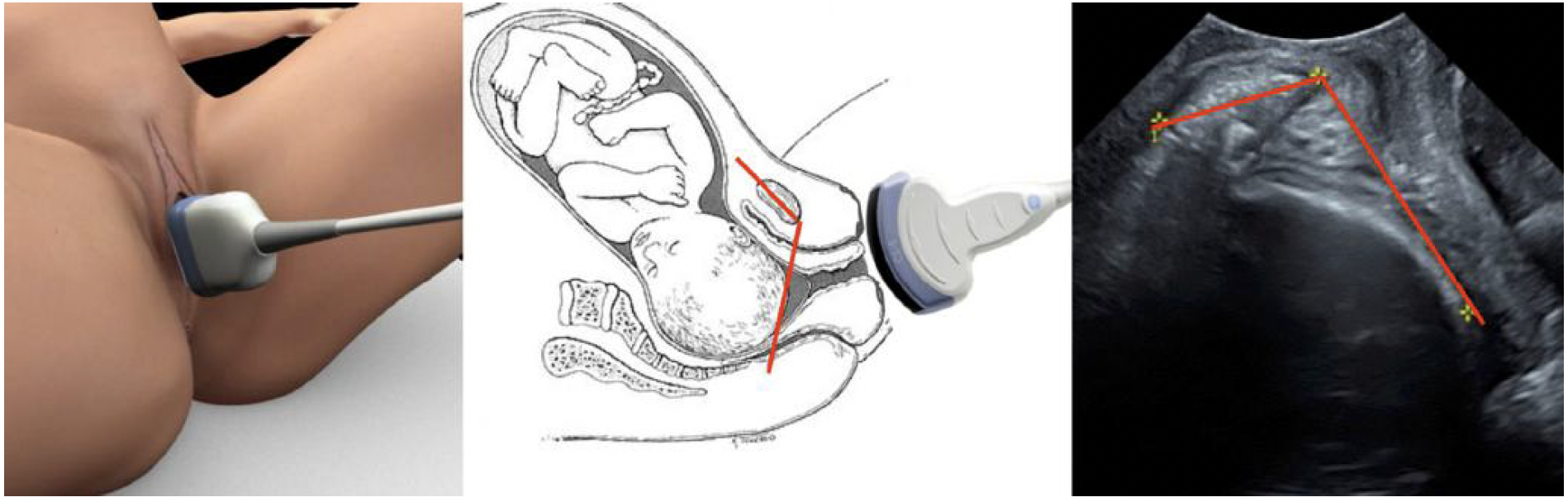
Table 1 Conversion between angle of progression (AoP) and
transperineal ultrasound (TPU) head station
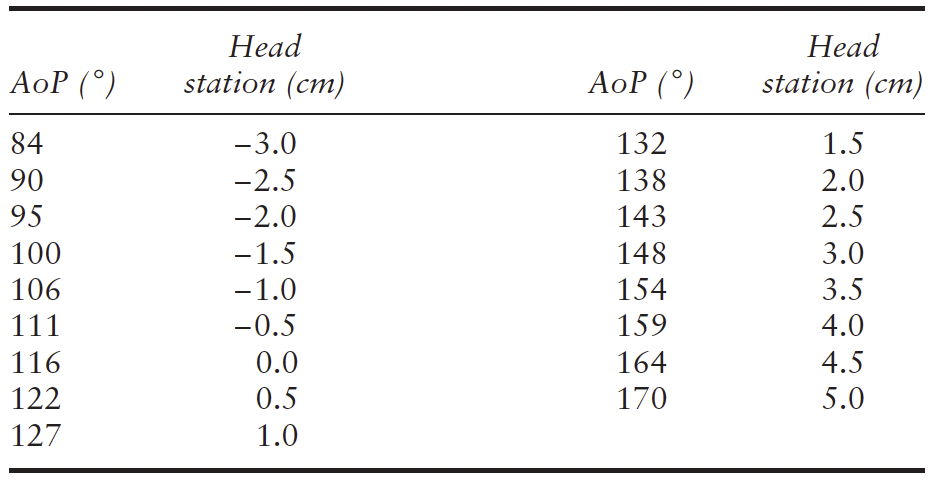 Adapted from Tutschek et al.41. TPU head station calculated using
formula obtained by regression of head station over angle of
progression (TPU head station (cm)=AoP (◦) 0.0937−10.911).
Adapted from Tutschek et al.41. TPU head station calculated using
formula obtained by regression of head station over angle of
progression (TPU head station (cm)=AoP (◦) 0.0937−10.911).
classified categorically as ‘head down’ (angle <0◦), ‘horizontal’
(angle 0◦–30◦) and ‘head up’ (angle >30◦). The
authors noted an easily recognizable change in head
direction as it descends towards the pelvic floor, from
downward to horizontal to upward. Head up immediately
before operative vaginal delivery (OVD) correlated
with a successful and relatively easy (few tractions)
procedure.
Sonographic head station. The transperineal ultrasound
head station expresses head station on the scale conventionally
used for palpatory assessment of progress of
labor (cm above or below the ischial spine plane) and
incorporates the curvature of the birth canal. It requires
assessment of: (i) the head direction (see above) and (ii)
the distance between the infrapubic plane (which is 3 cm
above the ischial plane) and the deepest presenting bony
part along the line of head direction (Figure 6). Transperineal
ultrasound head station has been compared with
other parameters of fetal head station. While it is more
complex to measure (requiring both angle and distance
measurements), it was found to correlate linearly with
the easily measurable AoP: the relationship between these
two parameters thus allows direct conversion of AoP measurements
into centimeters on the conventional palpation
scale (Table 1).
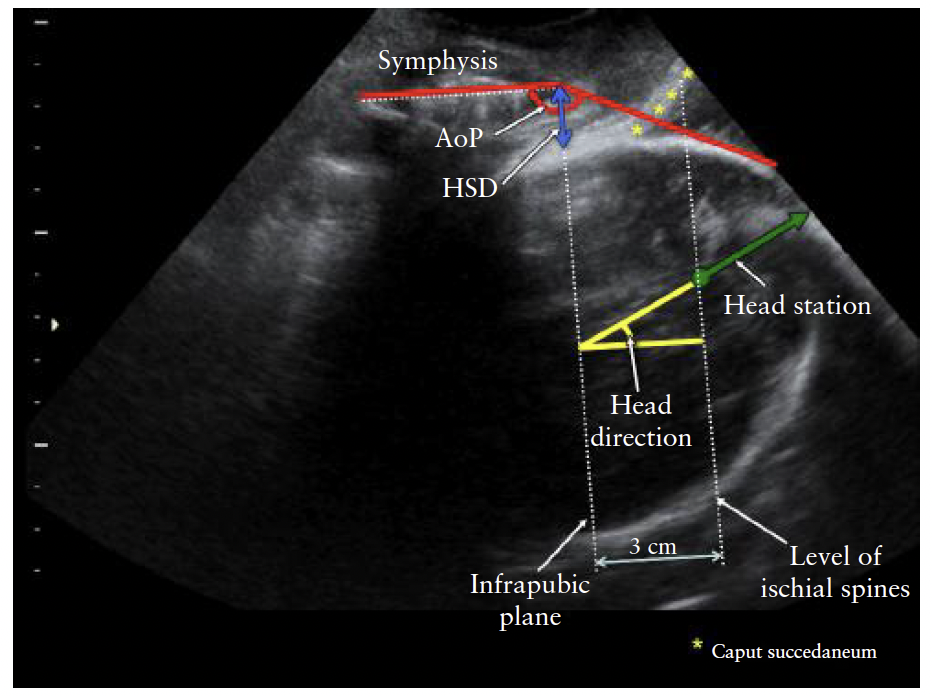 Figure 6 Transperineal ultrasound head station should be
measured along line of head direction. Angle of progression (AoP),
head–symphysis distance (HSD), and, as reference planes,
measurable infrapubic plane and inferred ischial plane, are also
shown (modified from Tutschek et al.32).
Figure 6 Transperineal ultrasound head station should be
measured along line of head direction. Angle of progression (AoP),
head–symphysis distance (HSD), and, as reference planes,
measurable infrapubic plane and inferred ischial plane, are also
shown (modified from Tutschek et al.32).
Head–perineum distance (HPD). HPD was first
described by Eggeb et al.34 (Figure 7). The transducer
should be placed between the labia majora (in the posterior
fourchette), and the soft tissue compressed completely
against the pubic bone. The transducer should be angled
until the skull contour is as clear as possible, indicating
that the ultrasound beam is perpendicular to the fetal
skull. HPD is measured in a frontal transperineal scan
as the shortest distance from the outer bony limit of the
fetal skull to the perineum. This distance represents the
part of the birth canal yet to be passed by the fetus.
Women do not find this compression of the soft tissue to
be painful36.
HPD cannot be compared directly with the clinical
assessment of fetal head station (from –5 to +5) because
HPD does not follow the curve of the birth canal36.
Tutschek et al.32 found head station 0 to correspond to a
HPD of 36 mm, Kahrs et al.47 found head station 0 to correspond
to a HPD of 35mm andMaticot-Baptista et al.85
found a HPD of 38mm to correspond to midcavity.
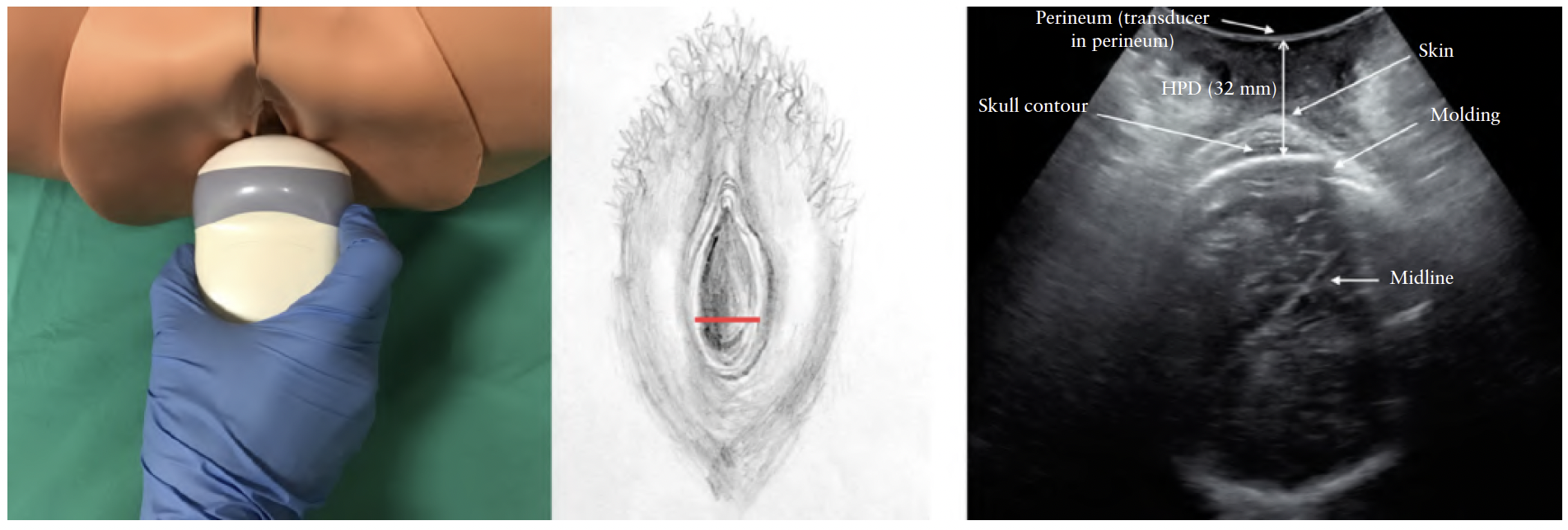
Limits of agreement for interobserver measurement variation
were reported as –8.5 to +12.3mm34.
Midline angle (MLA). MLA differs from the other parameters
as it utilizes the angle of head rotation as an indicator
of birth progress. First described by Ghi et al.31, it is measured
in the axial plane using a transperineal approach:
the echogenic line interposed between the two cerebral
hemispheres (midline) is identified, and MLA is the angle
between this line and the anteroposterior axis of thematernal
pelvis (Figure 8). They found a significant correlation
between head station assessed clinically and rotation as
represented by MLA. After excluding occiput posterior
cases, they found a rotation ≥45◦ to correspond to a
head station of ≤+2 cm in 70/71 (98.6%) cases and a
rotation <45◦ to correspond to a head station of≥+3 cm
in 41/49 (83.7%) cases (P<0.001) (LEVEL OF EVIDENCE:
2+). Although MLA was originally described as
an angle in relation to the maternal pelvis, head position
can be represented using positions on a clock face in the
same way as described for transabdominal imaging.
Additional parameters to assess fetal head station. Two
further parameters have been proposed to measure the
fetal head station in labor: progression distance (PD) and
head–symphysis distance (HSD). However, they have not
been applied widely in research studies and their clinical
usefulness is less well established than that of the other
parameters.
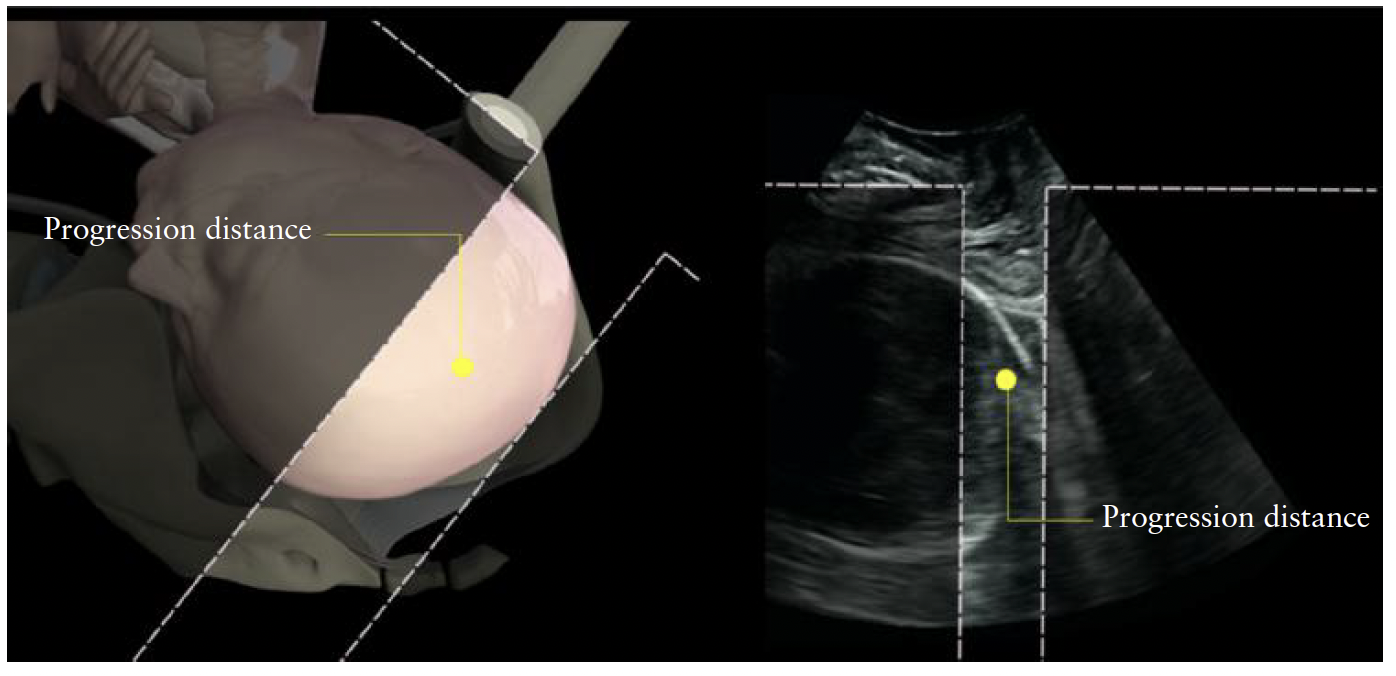
PD was first described as an objective measurement
of fetal head engagement, taken before onset of labor,
by Dietz and Lanzarone30. It is defined as the minimum
distance between the ‘infrapubic line’ and the presenting
part (defined as the most distal part of the hyperechogenic
curvature signifying the fetal skull) (Figure 9). Because
AoP is easier to measure than PD and accounts for the
curved nature of the birth canal, which PD does not, the
former should be preferred as a measure of head station.
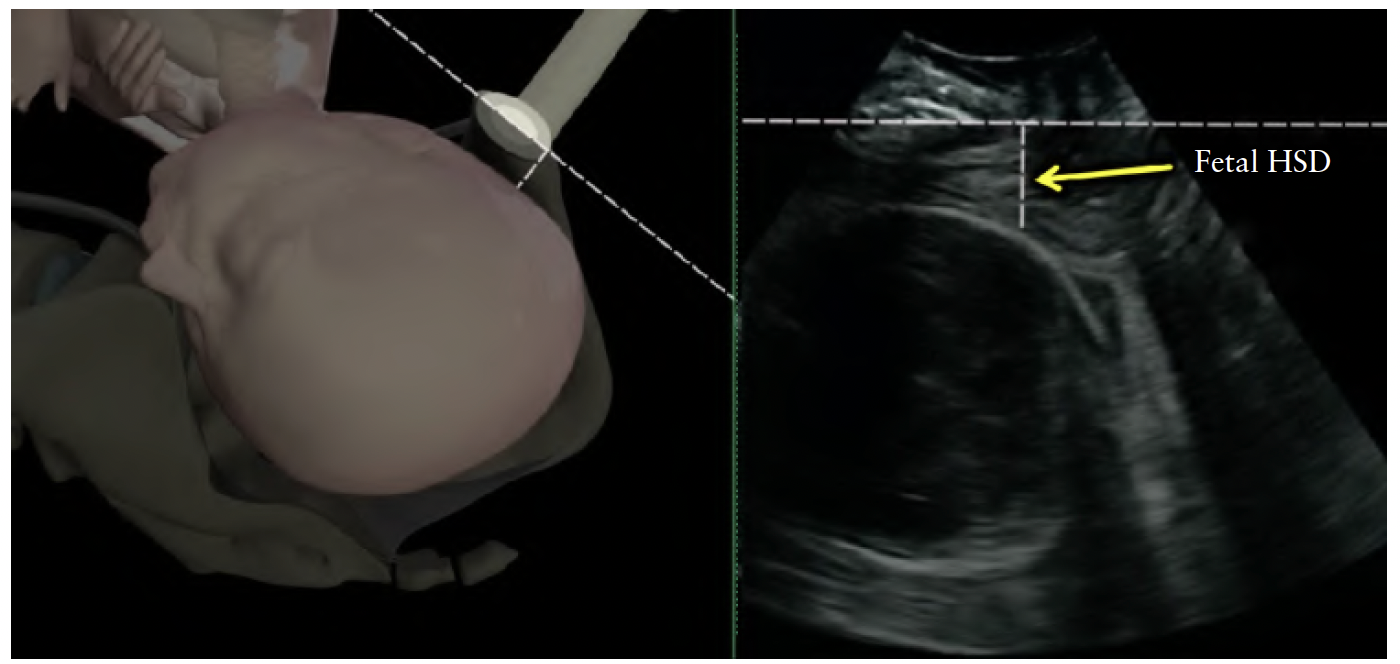
HSD is the distance between the lower edge of the
maternal symphysis pubis and the fetal skull, along the
infrapubic line (Figure 10). As the palpable space between
the fetal skull and the maternal symphysis pubis is used
widely in clinical practice as a proxy for fetal head station,
the HSD has been proposed by Youssef et al.51 as
an indirect marker of fetal head descent. In a cohort of
occiput-anterior fetuses this parameter has been proved
reproducible51, showing a linear negative correlation with
the palpated station and becoming progressively shorter
as the head descends towards the pelvic floor (LEVEL
OF EVIDENCE: 2+). Furthermore, HSD has been shown
to correlate with the other sonographic measurements
of fetal head station; it is correlated positively with HPD
and negatively with AoP32 (Figure 11). It can be measured
only at stations below the infrapubic line (i.e. ≥ –3 cm).
INDICATIONS FOR ULTRASOUND EVALUATION IN LABOR
- Slow progress or arrest of labor in the first stage
- Slow progress or arrest of labor in the second stage
- Ascertainment of fetal head position and station before considering or performing instrumental vaginal delivery
- Objective assessment of fetal head malpresentation
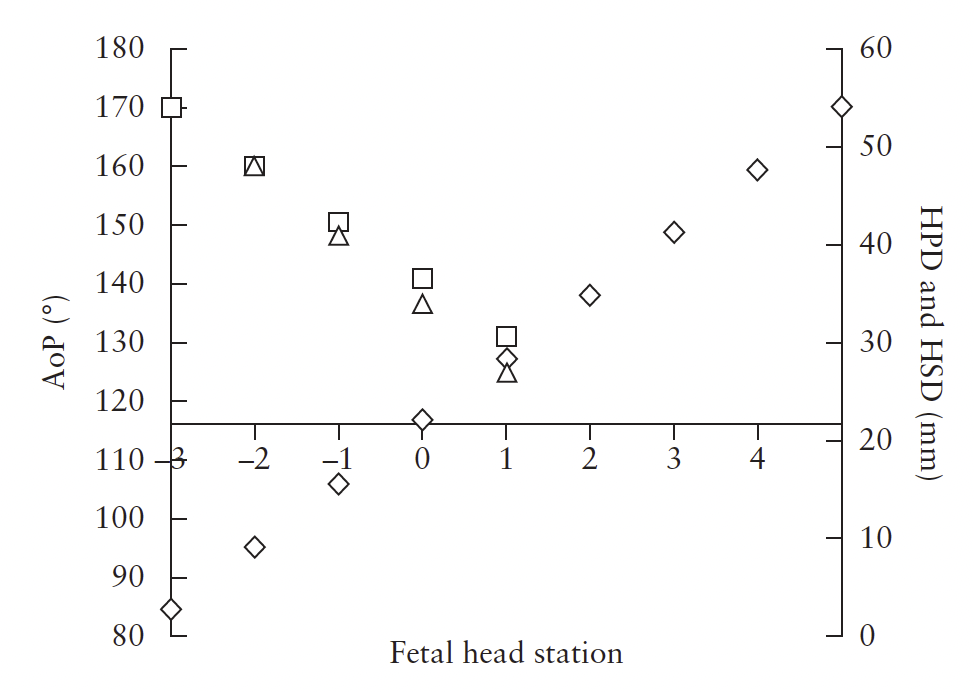 Figure 11 Correlation of transperineal ultrasound (TPU) parameters
representative of fetal head station: angle of progression
(AoP; ); head–perineum distance (HPD; ); and head–symphysis
distance (HSD; ). TPU head station is in cm above or below level
of ischial spines. Data are from Tutschek et al.32.
Figure 11 Correlation of transperineal ultrasound (TPU) parameters
representative of fetal head station: angle of progression
(AoP; ); head–perineum distance (HPD; ); and head–symphysis
distance (HSD; ). TPU head station is in cm above or below level
of ischial spines. Data are from Tutschek et al.32.
One study failed to demonstrate a benefit of routine
use of ultrasound in labor for determination of head
position (head station was not measured by ultrasound
in this study) among low-risk patients, in whom its use
was associated with a higher risk of Cesarean delivery86
(LEVEL OF EVIDENCE: 1–, GRADEOF RECOMMENDATION:
A).
Although ultrasound has been demonstrated to be more
accurate and reproducible than digital examination in
the determination of fetal head position and station in
labor, knowledge of these findings has not been shown to
improve the management of labor and delivery. Because
of the rarity of adverse perinatal and maternal outcomes
during labor, very large randomized studies would be
necessary to prove a clinical benefit of intrapartum sonography
for the fetus or the mother with respect to severe
perinatal or maternal morbidity. However, intrapartum
ultrasound allows more precise determination of position
and station and is more acceptable to women than digital
examination72. Its use may be endorsed under the following
circumstances as an adjunct to clinical examination.
Some consecutive studies have shown that HPD and AoP
are more accurate than digital examination in predicting
vaginal delivery in nulliparous womenwith prolonged first
stage of labor36,39 (LEVEL OF EVIDENCE: 2+, GRADE
OF RECOMMENDATION: B). In the largest multicenter
trial, conducted on 150 women39, if HPD was <40 mm,
the likelihood of Cesarean delivery was 7%, whereas it
went up to 82% if HPD was >50 mm. In the same study,
if AoP was >110◦, the likelihood of Cesarean delivery
was 12%, whereas this rose to 62% if AoP was <100◦.
In a study of the same population of 150 women
with prolonged first stage of labor37, the authors
showed that occiput-posterior position, compared with
non-occiput-posterior position, was significantly associated
with the risk of Cesarean section (38% vs 17%,
P= 0.01) (LEVEL OF EVIDENCE: 2+, GRADE OF
RECOMMENDATION: B).
Several case reports or small series76–80 have shown
that, in patients with prolonged first stage of labor, transabdominal
or transperineal ultrasound may identify as
a cause of labor arrest different types of head malpresentation,
including deflexed presentation (brow or face)
or asynclitism (LEVEL OF EVIDENCE: 3, GRADE OF
RECOMMENDATION: C).
There is a paucity of studies addressing specifically the
usefulness of ultrasound in predicting the chance of spontaneous
vaginal delivery compared with that of abdominal
delivery or OVD in patients with prolonged second stage.
In 62 women with prolonged second stage examined by
transperineal ultrasound, Masturzo et al.73 found that a
favorable head direction (head up) was associated with
spontaneous vaginal delivery in the majority (16/20; 80%)
of cases, in contrast to downward (4/20; 20%) or horizontal
(9/22; 41%) head direction (LEVEL OF EVIDENCE:
2+, GRADE OF RECOMMENDATION: B).
In a recent randomized controlled trial28, it was demonstrated
that ultrasound assessment in addition to digital
examination prior to instrumental vaginal delivery is
significantly more accurate compared with digital examination
alone in the diagnosis of fetal head position
(ultrasound diagnosis incorrect in 1.6% of cases, compared
with 20.2% in digital examination group) (LEVEL
OF EVIDENCE: 1–, GRADE OF RECOMMENDATION:
A). While the study did not show significant
differences in maternal or fetal morbidity, the main outcome
was the accuracy of determining fetal position, and
the study was not powered to detect differences in the
occurrence of adverse events87.
In their randomized controlled trial, Wong et al.88
demonstrated that when fetal head position is determined
by ultrasound compared with by palpation, placement
of the suction cup was significantly closer to the flexion
point (LEVEL OF EVIDENCE: 1–, GRADE OF RECOMMENDATION:
A).
Head direction predicts the outcome of instrumental
vaginal delivery42.When evaluated before vacuum extraction
in protracted labor, the head-up sign is a positive
predictor of success. Among 11 women with fetal head up
and an occiput-anterior position, all had successful simple
(5/11) or moderately difficult (6/11) vacuum extraction. In
contrast, among the six cases with occiput-anterior fetus
with head horizontal or down, only one vacuum extraction
was simple, and the only case of failed extraction was
observed in this group. The value of the head-up sign for
prediction of vaginal delivery as well as its good intra- and
interobserver agreement were subsequently confirmed by
others41 (LEVEL OF EVIDENCE: 3, GRADE OF RECOMMENDATION:
C).
AoP was investigated as a predictor of successful vacuum
delivery in 41 fetuses in occiput-anterior position. A
cut-off value of 120◦ was found to predict an easy and successful
vacuum extraction in 90% of cases43 (LEVEL OF
EVIDENCE: 2+, GRADE OF RECOMMENDATION:
B).
In 52 women with occiput-anterior fetus undergoing
vacuum delivery, the combination of head-up sign,
MLA<45◦ and AoP>120◦ were found to be significant
sonographic predictors of a successful procedure45.
Cuerva et al.46 assessed the role of ultrasound in
predicting the outcome of forceps delivery in 30
non-occiput-posterior fetuses. They found that the smaller
the AoP and the shorter the PD, the higher the risk of
failure. AoP<138◦ and PD<4.8 cm were the strongest
predictors of the nine complicated procedures (defined as
requiring more than three tractions, failed procedure, or
maternal or neonatal trauma) (LEVEL OF EVIDENCE:
2+, GRADE OF RECOMMENDATION: B).
A recent large study44 investigated the relationship
between vacuum extraction failure rate and AoP (immediately
prior to application of the instrument) in 235
women. In 30 (12%), the vacuum extraction failed,
while in the remaining 205 it was successful. Failed vacuum
delivery was associated with a significantly smaller
median AoP (136.6◦ vs 145.9◦); interestingly, the palpated
head station did not differ between the two groups
(2 vs 2 cm) (LEVEL OF EVIDENCE: 2+, GRADE OF
RECOMMENDATION: B).
In a European prospective study47, transperineal ultrasound
and the duration of vacuum extraction in a cohort
of women with slow progress in the second stage of
labor were assessed. Among the 222 women included,
the duration of the extraction procedure was significantly
shorter in women with HPD≤25 mm. The rate
of Cesarean delivery was significantly lower among
cases with HPD≤35mm compared with those with
HPD >35mm (3.9% vs 22.0%, P<0.01) and, if
HPD>35mm was combined with occiput-posterior position,
the rate of Cesarean delivery was 35%. Furthermore,
the incidence of umbilical artery pH<7.1 was significantly
higher in the infants which underwent vacuum
delivery with HPD >35 mm.
In a prospective cohort study including 659 women,
the HPD (in this study referred to as the perineum–skull
distance) was measured prior to OVD48. After adjustment
for parity, presentation type and fetal macrosomia,
HPD≥40mmwas significantly associated with the occurrence
of a difficult extraction (odds ratio, 2.38; 95%
CI, 1.51–3.74; P=0.0002). Based on receiver–operating
characteristics curve analysis, perineum–skull distance on
ultrasound was amore accurate predictor of difficultOVD
than was digital vaginal examination (P=0.036).
Deflexed cephalic presentation or asynclitism is a major cause of obstructed labor13,14, estimated to account for one-third of Cesarean deliveries for arrest of labor4–6,8–10,15–17. In these cases the diagnosis is based traditionally upon digital examination in labor89–91, although the use of ultrasound to support the clinical diagnosis has been reported recently76–80 (LEVEL OF EVIDENCE: 3, GRADE OF RECOMMENDATION: C).
SUMMARY
Ultrasound in active labor is not yet used widely, even though studies have shown that it is more precise and reproducible than clinical examination. Ultrasound allows objective measurement and precise documentation of findings obtained during the examination. Several sonographic parameters can be used during labor to assess mainly head station and position.
1. Head station can be measured objectively, for example
by AoP or HPD, to assess current status and as a baseline
for longitudinal measurements. It can also help
to predict whether OVD is likely to be successful.
Head station should be assessed transperineally, not
transabdominally. HPD is straightforward to measure
and is reproducible. AoP (in degrees) is equivalent to
head station expressed in centimeters, from –3 cm
to +5 cm (direct conversion is possible), and has
the potential to link ultrasound data to traditional
assessment by palpation. HPD and AoP/head station
correlate linearly (for high station, i.e. higher
than 0 to +1).
2. Head (and spine) position is assessed more accurately
by transabdominal ultrasound than by digital palpation.
Knowledge of head position in suspected delay or
arrest of labor is important. Before OVD, knowledge
of head position is essential.
3. MLA is assessed by transverse transperineal ultrasound
and may help to decide whether OVD can be
attempted safely.
4. Head direction is assessed by transperineal ultrasound
and may help to decide whether OVD can
be attempted safely.
There are two main situations in which ultrasound
assessment is likely to be of particular use in labor.
1. Suspected delay or arrest of first or second stage.
We recommend measurement of either AoP or HPD
transperineally and assessment of head position transabdominally.
2. Potential need for performance of OVD. We recommend
assessment of head position by transabdominal
ultrasound and suggest measurement of fetal head
station by transperineal ultrasound. The most reliable
sonographic parameters to predict outcome of the procedure
are HPD and AoP. MLA and/or head direction
may also be useful to predict further the likelihood of
success of the extraction.
GUIDELINE AUTHORS
This Guideline was produced on behalf of the International
Society of Ultrasound in Obstetrics and Gynecology
(ISUOG) by the following authors, and peer reviewed by
the Clinical Standards Committee.
D. Paladini, Fetal Medicine and Surgery Unit, Istituto G.
Gaslini, Genoa, Italy
G. Malinger, Division of Ultrasound in Obstetrics and
Gynecology, Lis Maternity Hospital, Tel Aviv Sourasky
Medical Centre, Sackler School of Medicine, Tel Aviv
University, Tel Aviv, Israel
R. Birnbaum, Division of Ultrasound in Obstetrics and
Gynecology, Lis Maternity Hospital, Tel Aviv Sourasky
Medical Centre, Sackler School of Medicine, Tel Aviv
University, Tel Aviv, Israel
A. Monteagudo, Carnegie Imaging for Women, Obstetrics,
Gynecology and Reproductive Science, Icahn School
of Medicine at Mount Sinai, New York, NY, USA
G. Pilu, Obstetric Unit, Department of Medical and
Surgical Sciences, University of Bologna, Bologna, Italy
L. J. Salomon, Hˆ opital Necker Enfants Malades, AP-HP,
and LUMIERE platform, EA 7328 Universit e de Paris,
Paris, France
I. E. Timor-Tritsch, Division of Obstetrical and Gynecological
Ultrasound, NYU School of Medicine, New York,
NY, USA
CITATION
This Guideline should be cited as: ‘Paladini D, MalingerG, Birnbaum R, Monteagudo A, Pilu G, Salomon LJ, Timor-Tritsch IE. ISUOG Practice Guidelines (updated): sonographic examination of the fetal central nervous system. Part 2: performance of targeted neurosonography. Ultrasound Obstet Gynecol 2021. https://doi.org/10.1002/uog.23616.
REFERENCES
1. Tagliabue G, Tessandori R, Caramaschi F, Fabiano S, Maghini A, Tittarelli A,
Vergani D, Bellotti M, Pisani S, Gambino ML, Frassoldi E, Costa E, Gada D,
Crosignani P, Contiero P. Descriptive epidemiology of selected birth defects, areas of
Lombardy, Italy, 1999. Popul Health Metr 2007; 5: 4.
2. Atta CA, Fiest KM, Frolkis AD, Jette N, Pringsheim T, St Germaine-Smith C,
Rajapakse T, Kaplan GG, Metcalfe A. Global Birth Prevalence of Spina Bifida by
Folic Acid Fortification Status: A Systematic Review and Meta-Analysis. Am J Public
Health 2016; 106: e24–34.
3. Myrianthopoulos NC. Epidemiology of central nervous system malformations. In
Handbook of Clinical Neurology. Vinken PJ, Bruyn GW (eds). Elsevier: Amsterdam,
1977; 139–171.
4. Salomon LJ, Alfirevic Z, Berghella V, Bilardo C, Hernandez-Andrade E, Johnsen SL,
Kalache K, Leung KY, Malinger G, Munoz H, Prefumo F, Toi A, Lee W, Committee
ICS. Practice guidelines for performance of the routine mid-trimester fetal ultrasound
scan. Ultrasound Obstet Gynecol 2011; 37: 116–126.
5. Malinger G, Paladini D, Haratz KK, Monteagudo A, Pilu GL, Timor-Tritsch IE.
ISUOG Practice Guidelines (updated): sonographic examination of the fetal central
nervous system. Part 1: performance of screening examination and indications for
targeted neurosonography. Ultrasound Obstet Gynecol 2020; 56: 476–484.
6. Malinger G, Birnbam R, Haratz KK. Dedicated neurosonography for recognition of
pathology associated with mild-to-moderate ventriculomegaly. Ultrasound Obstet
Gynecol 2020; 56: 319–323.
7. Monteagudo A, Timor-Tritsch IE, Mayberry P. Three-dimensional transvaginal
neurosonography of the fetal brain: ‘navigating’ in the volume scan. Ultrasound
Obstet Gynecol 2000; 16: 307–313.
8. Malinger G, Katz A, Zakut H. Transvaginal fetal neurosonography. Supratentorial
structures. Isr J Obstet Gynecol 1993; 4: 1–5.
9. Timor-Tritsch IE, Monteagudo A. Transvaginal fetal neurosonography: standardization
of the planes and sections by anatomic landmarks. Ultrasound Obstet Gynecol
1996; 8: 42–47.
10. Timor-Tritsch IE, Monteagudo A, Pilu G, Malinger G. Ultrasonography of the fetal
brain. McGraw-Hill: New York, 2012.
11. Paladini D, Donarini G, Rossi A. Indications for MRI in fetal isolated mild
ventriculomegaly . . . ‘And then, there were none’. Ultrasound Obstet Gynecol 2019;
54: 151–155.
12. Napolitano R, Molloholli M, Donadono V, Ohuma EO, Wanyonyi SZ, Kemp B,
Yaqub MK, Ash S, Barros FC, Carvalho M, Jaffer YA, Noble JA, Oberto M,
Purwar M, Pang R, Cheikh Ismail L, Lambert A, Gravett MG, Salomon LJ,
Bhutta ZA, Kennedy SH, Villar J, Papageorghiou AT, International F, Newborn
Growth Consortium for the 21st C. International standards for fetal brain structures
based on serial ultrasound measurements from Fetal Growth Longitudinal Study of
INTERGROWTH-21st Project. Ultrasound Obstet Gynecol 2020; 56: 359–370.
13. Droulle P, Gaillet J, Schweitzer M. [Maturation of the fetal brain. Echoanatomy:
normal development, limits and value of pathology]. J Gynecol Obstet Biol Reprod
1984; 13: 228–236.
14. Monteagudo A, Timor-Tritsch IE. Development of fetal gyri, sulci and fissures: a
transvaginal sonographic study. Ultrasound Obstet Gynecol 1997; 9: 222–228.
15. Cohen-Sacher B, Lerman-Sagie T, Lev D, Malinger G. Developmental milestones
of the fetal cerebral cortex. A longitudinal sonographic study. Ultrasound Obstet
Gynecol 2006; 27: 494–502.
16. Toi A, Chitayat D, Blaser S. Abnormalities of the foetal cerebral cortex. Prenat Diagn
2009; 29: 355–371.
17. Poon LC, Sahota DS, Chaemsaithong P, Nakamura T, Machida M, Naruse K, Wah
YM, Leung TY, Pooh RK. Transvaginal three-dimensional ultrasound assessment of
Sylvian fissures at 18–30 weeks’ gestation. Ultrasound Obstet Gynecol 2019; 54:
190–198.
18. Acanfora MM, Stirnemann J, Marchitelli G, Salomon LJ, Ville Y. Ultrasound
evaluation of development of olfactory sulci in normal fetuses: a possible role in
diagnosis of CHARGE syndrome. Ultrasound Obstet Gynecol 2016; 48: 181–184.
19. Perlitz Y, Izhaki I, Ben-Ami M. Sonographic evaluation of the fetal conus medullaris
at 20 to 24 weeks’ gestation. Prenat Diagn 2010; 30: 862–864.
20. MottetN, Saada J, Jani J,Martin A, RiethmullerD, Zerah M, Benachi A. Sonographic
Evaluation of Fetal Conus Medullaris and Filum Terminale. Fetal Diagn Ther 2016;
40: 224–230.
21. Rodriguez MA, Prats P, Rodriguez I, Comas C. Prenatal Evaluation of the Fetal
Conus Medullaris on a Routine Scan. Fetal Diagn Ther 2016; 39: 113–116.
22. Fratelli N, Taddei F, Prefumo F, Franceschetti L, Farina G, Frusca T. Interobserver
reproducibility of transabdominal 3-dimensional sonography of the fetal brain.
J Ultrasound Med 2009; 28: 1009–1013.
23. Maiz N, Alonso I, Belar M, Burgos J, Irasarri A, Molina FS, de Paco C, Pijoan JI,
Plasencia W, Rodo C, Rodriguez MA, Tajada M, Tubau A. Three dimensional
ultrasonography for advanced neurosonography (Neurosofe-3d). Analysis of
acquisition-related factors influencing the quality of the brain volumes. Prenat
Diagn 2016; 36: 1054–1060.
24. Buyukkurt S, Binokay F, Seydaoglu G, Gulec UK, Ozgunen FT, Evruke C, Demir C.
Prenatal determination of the upper lesion level of spina bifida with three-dimensional
ultrasound. Fetal Diagn Ther 2013; 33: 36–40.
25. Bronshtein M, Blumenfeld Z. Transvaginal sonography-detection of findings
suggestive of fetal chromosomal anomalies in the first and early second trimesters.
Prenat Diagn 1992; 12: 587–593.
26. Pooh RK. Neurosonoembryology by three-dimensional ultrasound. Semin Fetal
Neonatal Med 2012; 17: 261–268.
27. Rottem S, Bronshtein M, Thaler I, Brandes JM. First trimester transvaginal
sonographic diagnosis of fetal anomalies. Lancet 1989; 1: 444–445.
28. Votino C, Kacem Y, Dobrescu O, Dessy H, Cos T, Foulon W, Jani J. Use of
a high-frequency linear transducer and MTI filtered color flow mapping in the
assessment of fetal heart anatomy at the routine 11 to 13 + 6-week scan: a
randomized trial. Ultrasound Obstet Gynecol 2012; 39: 145–151.
29. Chaoui R, Nicolaides KH. From nuchal translucency to intracranial translucency:
towards the early detection of spina bifida. Ultrasound Obstet Gynecol 2010; 35:
133–138.
30. Salomon LJ, Alfirevic Z, Bilardo CM, Chalouhi GE, Ghi T, Kagan KO, Lau
TK, Papageorghiou AT, Raine-Fenning NJ, Stirnemann J, Suresh S, Tabor A,
Timor-Tritsch IE, Toi A, Yeo G. ISUOG practice guidelines: performance of
first-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol 2013; 41: 102–113.
31. Paladini D, Donarini G, Parodi S, Chaoui R. Differentiating features of posterior
fossa at 12-13 weeks’ gestation in fetuses with Dandy-Walker malformation and
Blake’s pouch cyst. Ultrasound Obstet Gynecol 2019; 53: 850–852.
32. Chen FC, Gerhardt J, Entezami M, Chaoui R, Henrich W. Detection of Spina Bifida
by First Trimester Screening - Results of the Prospective Multicenter Berlin IT-Study.
Ultraschall Med 2017; 38: 151–157.
33. Meller C, Aiello H, Otano L. Sonographic detection of open spina bifida in the first
trimester: review of the literature. Childs Nerv Syst 2017; 33: 1101–1106.
34. Chaoui R, Benoit B, Entezami M, Frenzel W, Heling KS, Ladendorf B, Pietzsch V,
Sarut Lopez A, Karl K. Ratio of fetal choroid plexus to head size: simple sonographic
marker of open spina bifida at 11–13weeks’ gestation. Ultrasound Obstet Gynecol
2020; 55: 81–86.
35. Ushakov F, Sacco A, Andreeva E, Tudorache S, Everett T, David AL, Pandya PP.
Crash sign: new first-trimester sonographicmarker of spina bifida. Ultrasound Obstet
Gynecol 2019; 54: 740–745.
36. Volpe P, Persico N, Fanelli T, De Robertis V, D’Alessandro J, Boito S, Pilu G,
Votino C. Prospective detection and differential diagnosis of cystic posterior fossa
anomalies by assessing posterior brain at 11–14 weeks. Am J Obstet Gynecol MFM
2019; 1: 171–183.
37. Malinger G, Lev D, Lerman-Sagie T. The fetal cerebellum. Pitfalls in diagnosis and
management. Prenat Diagn 2009; 29: 372–380.
38. Birnbaum R, Barzilay R, Brusilov M, Wolman I, Malinger G. The normal
cavum veli interpositi at 14-17 weeks: three-dimensional and Doppler transvaginal
neurosonographic study. Ultrasound Obstet Gynecol 2020. DOI: 10.1002/uog
.22176.
39. Birnbaum R, Barzilay R, Brusilov M, Wolman I, Malinger G. The early pattern of
human corpus callosum development: A transvaginal 3D neurosonographic study.
Prenat Diagn 2020; 40: 1239–1245.
40. Babcook CJ, Chong BW, Salamat MS, Ellis WG, Goldstein RB. Sonographic anatomy
of the developing cerebellum: normal embryology can resemble pathology. AJR Am
J Roentgenol 1996; 166: 427–433.
41. Contro E, Volpe P, De Musso F, Muto B, Ghi T, De Robertis V, Pilu G. Open fourth
ventricle prior to 20 weeks’ gestation: a benign finding? Ultrasound Obstet Gynecol
2014; 43: 154–158.
42. Prayer D, Malinger G, Brugger PC, Cassady C, De Catte L, De Keersmaecker B,
Fernandes GL, Glanc P, Goncalves LF, Gruber GM, Laifer-Narin S, LeeW,Millischer
AE, Molho M, Neelavalli J, Platt L, Pugash D, Ramaekers P, Salomon LJ, Sanz M,
Timor-Tritsch IE, Tutschek B, Twickler D, Weber M, Ximenes R, Raine-Fenning N.
ISUOG Practice Guidelines: performance of fetal magnetic resonance imaging.
Ultrasound Obstet Gynecol 2017; 49: 671–680.
43. Malinger G, Ben-Sira L, Lev D, Ben-Aroya Z, Kidron D, Lerman-Sagie T. Fetal
brain imaging: a comparison between magnetic resonance imaging and dedicated
neurosonography. Ultrasound Obstet Gynecol 2004; 23: 333–340.
44. Paladini D, Quarantelli M, Sglavo G, Pastore G, Cavallaro A, D’Armiento
MR, Salvatore M, Nappi C. Accuracy of neurosonography and MRI in clinical
management of fetuses referred with central nervous system abnormalities.
Ultrasound Obstet Gynecol 2014; 44: 188–196.
45. Malinger G, Paladini D, Pilu G, Timor-Tritsch IE. Fetal cerebral magnetic resonance
imaging, neurosonography and the brave new world of fetal medicine. Ultrasound
Obstet Gynecol 2017; 50: 679–680.
46. Di Mascio D, Sileo FG, Khalil A, Rizzo G, Persico N, Brunelli R, Giancotti A,
Panici PB, Acharya G, D’Antonio F. Role of magnetic resonance imaging in fetuses
with mild or moderate ventriculomegaly in the era of fetal neurosonography:
systematic review and meta-analysis. Ultrasound Obstet Gynecol 2019; 54:
164–171.
APPENDIX 1 Grades of recommendation and levels of evidence used in ISUOG Guidelines
Classification of evidence levels
| 1++ | High-quality meta-analyses, systematic reviews of randomized controlled trials or randomized controlled trials with very low risk of bias |
| 1+ | Well-conducted meta-analyses, systematic reviews of randomized controlled trials or randomized controlled trials with low risk of bias |
| 1- | Meta-analyses, systematic reviews of randomized controlled trials or randomized controlled trials with high risk of bias |
| 2++ | High-quality systematic reviews of case–control or cohort studies or high-quality case–control or cohort studies with very low risk of confounding, bias or chance and high probability that the relationship is causal |
| 2+ | Well-conducted case–control or cohort studies with low risk of confounding, bias or chance and moderate probability that the relationship is causal |
| 2- | Case–control or cohort studies with high risk of confounding, bias or chance and significant risk that the relationship is not causal |
| 3 | Non-analytical studies, e.g. case reports, case series |
| 4 | Expert opinion |
Grades of recommendation
| A | At least one meta-analysis, systematic review or randomized controlled trial rated as 1++ and applicable directly to the target population; or a systematic review of randomized controlled trials or a body of evidence consisting principally of studies rated as 1+ applicable directly to the target population and demonstrating overall consistency of results |
| B | Body of evidence including studies rated as 2++ applicable directly to the target population and demonstrating overall consistency of results; or extrapolated evidence from studies rated as 1++ or 1+ |
| C | Body of evidence including studies rated as 2+ applicable directly to the target population and demonstrating overall consistency of results; or extrapolated evidence from studies rated as 2++ |
| D | Evidence level 3 or 4; or evidence extrapolated from studies rated as 2+ |
| Good practice point | Recommended best practice based on the clinical experience of the guideline development group |
