Published online in Wiley Online Library (wileyonlinelibrary.com). DOI: 10.1002/uog.22145
ISUOG Practice Guidelines (updated): sonographic examination of the fetal central nervous system. Part 1: performance of screening examination and indications for targeted neurosonography
The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) is a scientific organization that encourages sound clinical practice, and high-quality teaching and research related to diagnostic imaging in women’s healthcare. The ISUOG Clinical Standards Committee (CSC) has a remit to develop Practice Guidelines and Consensus Statements as educational recommendations that provide healthcare practitioners with a consensus-based approach, from experts, for diagnostic imaging. They are intended to reflect what is considered by ISUOG to be the best practice at the time at which they are issued. Although ISUOG has made every effort to ensure that Guidelines are accurate when issued, neither the Society nor any of its employees or members accepts any liability for the consequences of any inaccurate or misleading data, opinions or statements issued by the CSC. The ISUOG CSC documents are not intended to establish a legal standard of care, because interpretation of the evidence that underpins the Guidelines may be influenced by individual circumstances, local protocol and available resources. Approved Guidelines can be distributed freely with the permission of ISUOG ([email protected]).
Introduction
Central nervous system (CNS) malformations are some
of the most common congenital abnormalities. Neural
tube defects are the most frequent CNS malformations
and amount to about one to two cases per 1000 births.
The incidence of intracranial abnormalities with an intact
neural tube is uncertain, as most of these abnormalities
probably go undetected at birth and manifest only in later
life. However, long-term follow-up studies suggest that
the incidence may be as high as one in 100 births1.
Ultrasound has been used for nearly 30 years as the
main modality to help diagnose fetal CNS anomalies. The
aim of these Guidelines is to review, describe and update
the technical aspects of the screening evaluation of the
fetal brain to be performed as part of the midtrimester
anomaly scan, which is referred to in this document as
a ‘screening examination’. This Guideline also presents
the indications for the detailed evaluation of the fetal
CNS, which constitutes ‘targeted fetal neurosonography’,
a dedicated examination of the fetal brain and spine that
requires specific expertise and sophisticated ultrasound
equipment. This examination is described in Part 2 of this
Guideline, in which we also discuss the indications for
fetal brain magnetic resonance imaging (MRI). Details of
the grades of recommendation and levels of evidence used
in this Guideline are given in Appendix 1.
General considerations
Gestational age
Recommendation
Examiners involved in screening for CNS abnormalities
should be familiar with normal CNS appearance at
different gestational ages (GOODPRACTICE POINT).
The appearance of the brain and the spine changes
throughout gestation. To avoid diagnostic errors, it is
important to be familiar with normal CNS appearance
at different gestational ages (Figure 1), although most
efforts to diagnose CNS anomalies are focused around
midgestation2. Hence, it is recommended that this Guideline
is applied during the midtrimester anomaly scan.
Guideline
is applied during the midtrimester anomaly scan.
However, during the last decade, it has become
evident that an increasing number of CNS and neural
tube abnormalities, mainly dorsal and rhombencephalic
induction defects, may be visible from the end of the first
trimester3–9. Although these are in the minority, they are
usually severe and therefore deserve special consideration.
While early examination of the CNS requires certain
skills, it is always worthwhile paying particular attention
to the fetal head and brain at early gestational ages. The
advantage of early fetal neurosonography at 12–15weeks
is that the bones are thin and the brain may be evaluated
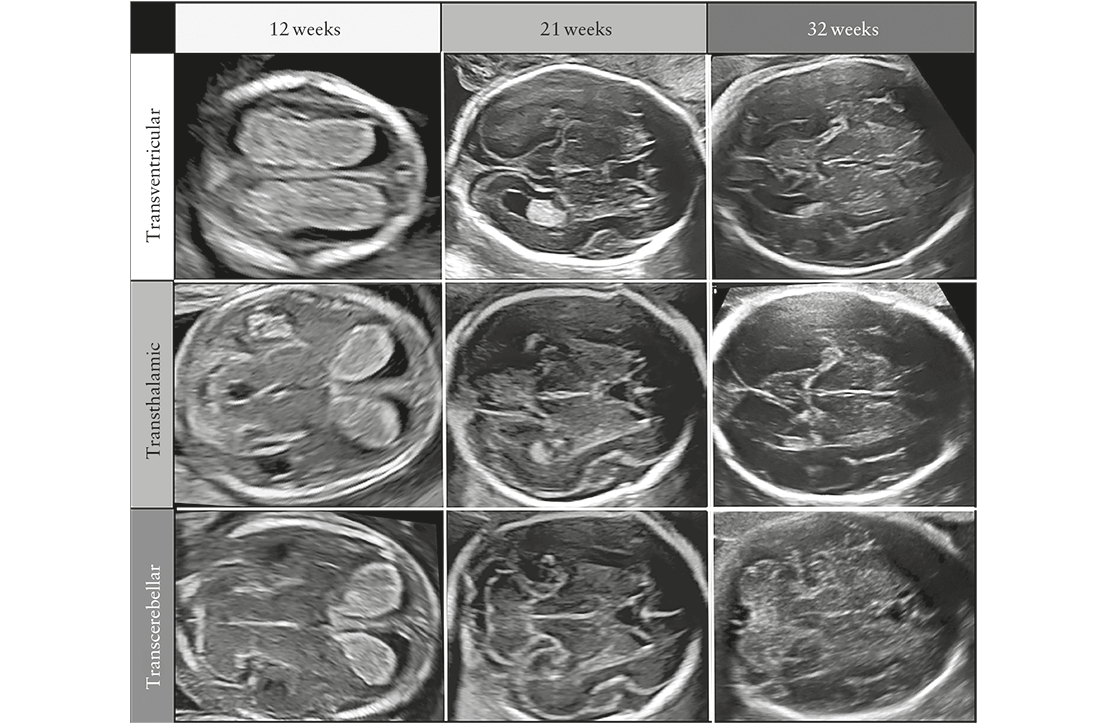
from almost all angles, especially with a high-frequency transvaginal transducer. Generally, a satisfactory evaluation of the fetal CNS can be performed from the end of the first trimester. As pregnancy advances, visualization of the intracranial structures becomes more difficult due to advanced ossification of the calvarium.
Technical factors
Ultrasound transducers
High-frequency ultrasound transducers increase spatial resolution but decrease the penetration of the sound beam. The choice of optimal transducer and operating frequency is influenced by a number of factors, including maternal habitus, fetal position, gestational age and the approach used. Most screening examinations are performed satisfactorily with a 3–5-MHz transabdominal transducer, although recent wideband transducers can also be employed advantageously.
Imaging parameters
The examination is performed with grayscale twodimensional ultrasound. Harmonic and crossbeam imaging, as well as speckle-reduction filters, may enhance visualization of subtle anatomic details and in patients who scan poorly, for example those with increased body mass index or abdominal scarring.
Screening examination of fetal brain after 18 weeks
Qualitative evaluation
Recommendation
Transabdominal sonography is the technique of choice
for the screening examination of the fetal CNS during
the midtrimester scan in low-risk pregnancies. This
examination should include evaluation of the fetal head
and spine (GOODPRACTICE POINT).
The fetal CNS screening examination during the
midtrimester scan in low-risk pregnancies should include
evaluation of the fetal head and spine, using transabdominal
sonography. Evaluation of two axial planes allows
visualization of the relevant cerebral structures to assess
the anatomic integrity of the fetal brain10. These planes are
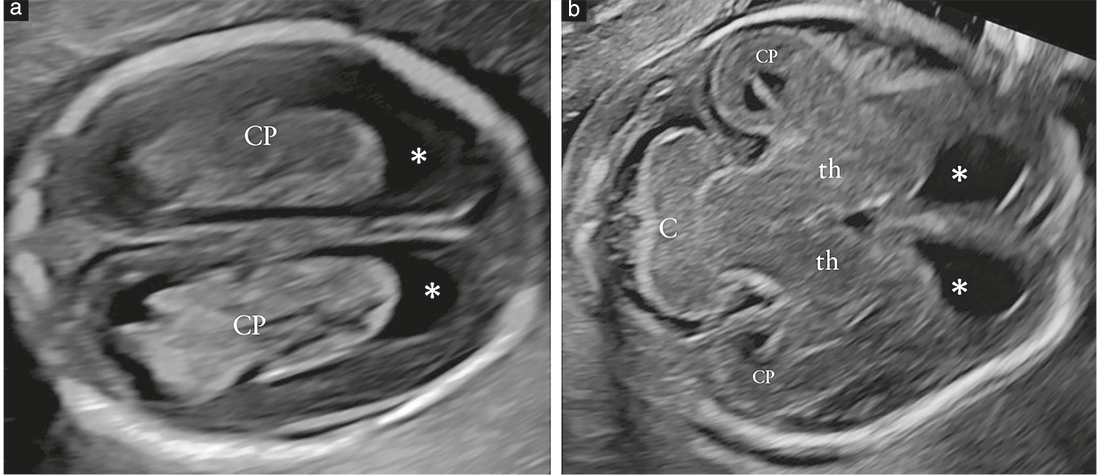
commonly referred to as the transventricular (Figure 2a)
and transcerebellar (Figure 2b) planes. A third plane, the
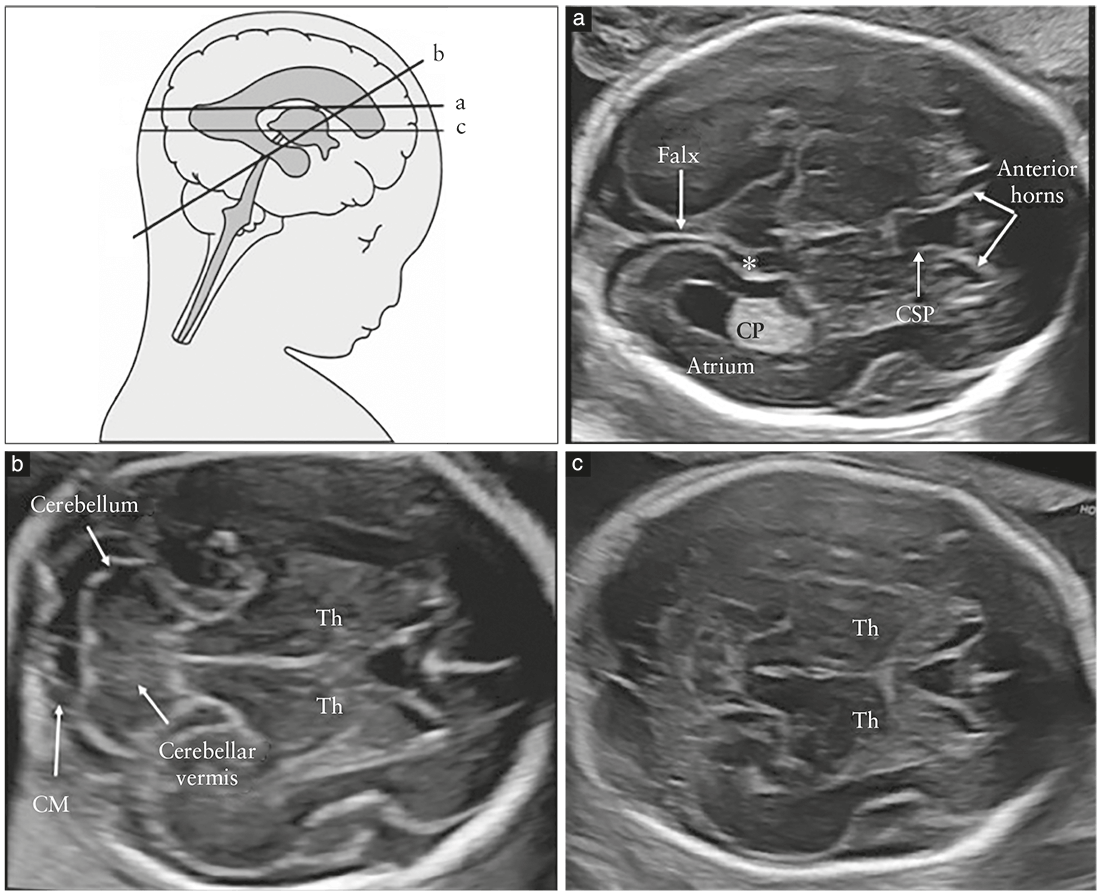
Table 1 Structures usually noted on screening ultrasound examination of fetal central nervous system
- Head shape
- Lateral ventricles
- Cavum septi pellucidi
- Thalami
- Cerebellum
- Cisterna magna
- Spine
so-called transthalamic plane (Figure 2c), is frequently added, mostly for the purpose of biometry. Structures that should be noted in the routine examination include the lateral ventricles, the cerebellum and cisterna magna, and the cavum septi pellucidi (CSP). Head shape and brain texture should also be noted on these views (Table 1).
Transventricular plane (Figure 2a)
Recommendation
In the transventricular plane, the aspect of the atrium
distal to the transducer and the presence of the
CSP should be assessed and documented (GOODPRACTICE POINT).
The transventricular plane demonstrates the anterior
and posterior portions of the lateral ventricles. The
anterior portion (frontal or anterior horns) appears as
two comma-shaped, fluid-filled structures. They have a
well-defined lateral wall and are separated medially by
the CSP. The CSP is a fluid-filled cavity between two thin
membranes. In late gestation or the early neonatal period,
these membranes usually fuse to become the septum
pellucidum. The CSP becomes visible between 17 and
20 weeks and disappears near term. Using transabdominal
ultrasound, it should always be demonstrable between
17–20 and 37 weeks, or at a biparietal diameter (BPD)
of 44–88mm11. Failure to demonstrate the CSP prior
to 16 weeks or later than 37 weeks is a normal finding;
rarely, absence of fluid in the CSP is seen in completely
normal fetuses12. The importance of visualizing the CSP
between 17 and 37 gestational weeks is due to the fact
that its non-visualization or an abnormal appearance is
associated with commissural anomalies, which may be an
indirect sign of corpus callosal agenesis on screening views
(usually in conjunction with a tear-shaped appearance
of the lateral ventricles, known as colpocephaly13).
Failure to visualize the membranes of the septum
pellucidum is highly suspicious for the presence of
a number of severe cerebral malformations, such as
holoprosencephaly, severe hydrocephaly and septo-optic
dysplasia14. Recently, an abnormal shape of the CSP has
been described as a relatively reliable marker of partial
agenesis of the corpus callosum15,16.
From about 16 weeks, the posterior portion of the
lateral ventricles (also referred to as occipital horns) is,
in reality, a complex formed by the atrium that continues
posteriorly into the occipital horn. The atrium is characterized
by the presence of the glomus of the choroid
plexus, which is highly echogenic, while the occipital horn
is filled with cerebrospinal fluid. Particularly in the second
trimester of gestation, both medial and lateral walls of
the ventricle are parallel to the midline and are therefore
well-depicted sonographically as well-demarcated
echogenic lines. Under normal conditions, the glomus
of the choroid plexus completely fills the cavity of the
ventricle at the level of the atrium, being in close contact
with the medial and lateral walls, although in some
normal cases a small amount of fluid may be present
between the medial wall and the choroid plexus17–20.
It should be noted that, due to artifacts in the near field
of the image, caused by shadowing from the proximal
parietal bone, in the standard transventricular plane, only
the hemisphere and the lateral ventricle on the far side
of the transducer are usually visualized clearly. However,
most severe cerebral lesions are bilateral or associated with
a significant deviation or distortion of the midline echo,
and it has been suggested that, in screening examinations,
symmetry of the brain can be assumed.
Transcerebellar plane (Figure 2b)
Recommendation
In the transcerebellar plane, the presence and shape of
the cerebellum, as well as the presence of cerebrospinal
fluid in the cisterna magna, should be assessed and
documented (GOODPRACTICE POINT).
The transcerebellar plane is slightly caudal to the transventricular
one, and it is usually obtained with slight posterior
tilting of the transducer. It is used to visualize the thalami,
cerebellum and cisterna magna. The cerebellum appears as
a butterfly-shaped structure formed by the round cerebellar
hemispheres joined in the middle by the slightly more
echogenic cerebellar vermis. The cisterna magna, or cisterna
cerebellomedullaris, is a fluid-filled space posterior
to the cerebellum. It normally contains thin septations,
which are not usually demonstrated in the presence of
pathology21. In the second half of gestation, the anteroposterior
diameter of the cisterna magna remains stable
and should not exceed 10mm10. Before 19–20 gestational
weeks, the cerebellar vermis has not yet completely covered
the fourth ventricle, and this unusual appearance
may give the false impression of a defect of the vermis. As
a rule of thumb, by 19 gestational weeks, there should be
no midline fluid-filled space between the two cerebellar
hemispheres; should this finding, referred to as ‘keyhole
sign’, be detected, it may be associated with an anomaly of
the cerebellar vermis and the fetus should be referred for
neurosonography22. Care should be taken to avoid ‘overtilting’
of the probe, since this will increase the likelihood
of false-positive diagnosis of a vermian anomaly.
Transthalamic plane (Figure 2c)
Commonly referred to as the transthalamic or BPD plane, a third scanning plane, obtained parallel but caudal to the transventricular plane, is also frequently used in the sonographic assessment of the fetal head. The anatomic landmarks include, from anterior to posterior, the frontal horns of the lateral ventricles, the CSP, the thalami and the hippocampal gyri23. This plane is used for biometry of the fetal head. It is easier to identify in late gestation and allows more reproducible measurements than does the transventricular plane24.
Fetal spine
Recommendation
When technically feasible, a longitudinal section of the
fetal spine should be obtained, in order to screen for
open and closed spinal dysraphism (GOODPRACTICE POINT).
Technical advice
Up to 97% of cases of open spina bifida present
with the so-called ‘banana sign’, which is due to
Chiari-II malformation25 (GRADE OF RECOMMENDATION: C).
Detailed examination of the fetal spine requires expertise
and meticulous scanning, and the results are heavily
dependent on the fetal position. Therefore, a full and
detailed evaluation of the fetal spine in every plane
is not part of the screening examination. One of the
most frequent severe spinal abnormalities, open spina
bifida, is usually associated with abnormal intracranial
anatomy: up to 97% of cases present with the so-called
‘banana sign’, which is due to Chiari-II malformation25.
However, a longitudinal section of the fetal spine should
be sought4 if technically feasible, because it may reveal, at
least in some cases, other spinal malformations, including
vertebral abnormalities and sacral agenesis, although the
latter diagnosis may be challenging even for experts,
due to the physiological non-ossification of the caudal
spine in the mid trimester26. Under normal conditions, a
sagittal section of the spine at 18–24 gestational weeks
demonstrates the three ossification centers of the vertebrae
(one inside the body and one on each side at the junction
between the lamina and pedicle) that surround the neural
canal, and that appear as either two or three parallel lines,
depending on the orientation of the ultrasound beam
(Figure 3). The three ossification nuclei are best visualized
on an axial view of individual vertebrae (Figure 4). In
addition, an attempt should be made to demonstrate the
intactness of the skin overlying the spine, on either a
transverse or a longitudinal view.
Quantitative evaluation
Recommendation
The following measurements represent an integral part
of sonographic screening for CNS malformations: atrial
width and transverse cerebellar diameter. Additional
measurements usually performed for general biometry
purposes (BPD and head circumference (HC)) are also
part of the examination, since they may, in some cases,
reveal proliferation abnormalities (e.g. microcephaly or
macrocephaly) (GOOD PRACTICE POINT).
Technical advice
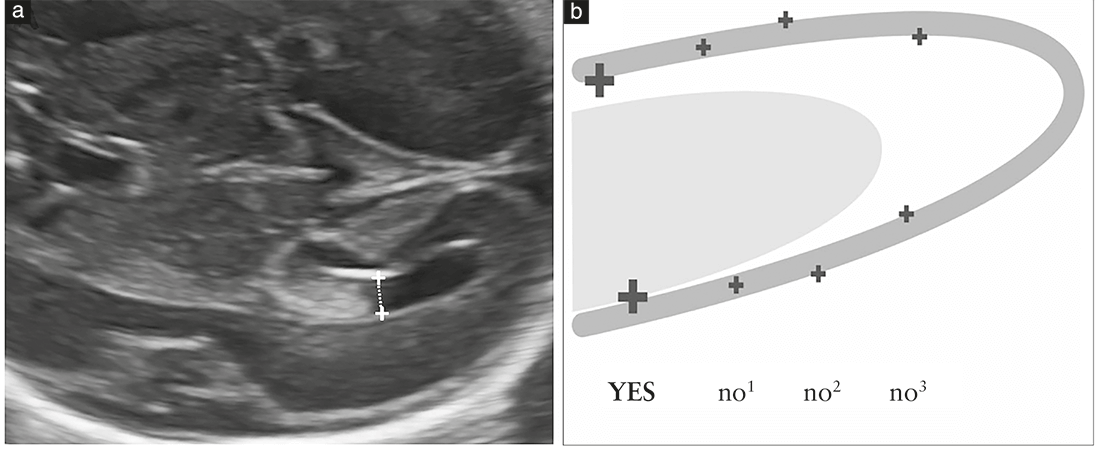
The atrial width should be measured inner-to-inner and
should be <10mm throughout pregnancy (GRADE OF
RECOMMENDATION: C).
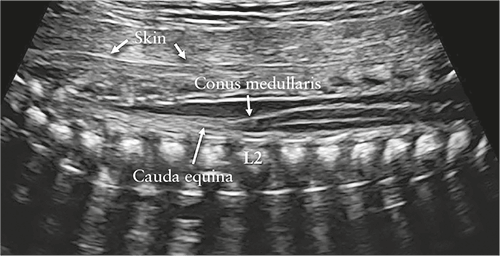
Figure 3 Sagittal view of lower thoracic and sacral fetal spine.
Using unossified spinous process of vertebrae as acoustic window,
contents of neural canal are demonstrated. Conus medullaris is
clearly demonstrated and is normally located at level of L2 in
midgestation. Its sharp end should point anteriorly, to vertebral
body, with fluid filling neural canal posteriorly. Note intact skin
observed as hyperechogenic line along fetal back.
Biometry is an essential part of sonographic examination
of the fetal head. In the second-trimester anomaly
scan, a standard examination includesmeasurement of the
BPD, HC, internal diameter of the atrium and transverse
cerebellar diameter. The cisterna magna depth should be
measured if this structure is visually thinner or wider
than normal on qualitative assessment of the posterior
fossa.
BPD and HC are commonly used for assessing fetal age
and growth and may also be useful to identify some
cerebral anomalies. They may be measured either in
the transventricular plane or in the transthalamic plane.
There are various techniques for measuring BPD. Most
frequently, the calipers are positioned outside the fetal
calvarium (so-called ‘outer-to-outer’ measurement)24.
However, some commonly used charts were produced
using an outer-to-inner technique, to avoid artifacts
generated by the distal echo of the calvarium, an issue
that is less relevant now, with modern transducers, than
it was several years ago23. These two approaches to
measurement result in a difference of a few millimeters,
which may be clinically relevant in early gestation. It is
important, therefore, to know the technique that was used
to construct the reference charts that one uses. HC can be
measured directly, with the ellipse method, by placing the
ellipse around the outer outline of the calvarium echoes.
Alternatively, it can be calculated after measuring the BPD
and occipitofrontal diameter (OFD), using the equation:
HC=1.62×(BPD+OFD). The BPD/OFD ratio is usually
70–85%. However,molding of the fetal head, particularly
in early gestation, is frequent, and fetuses in breech
presentation may show some degree of dolicocephaly.
It is not appropriate to use HC nomograms intended for
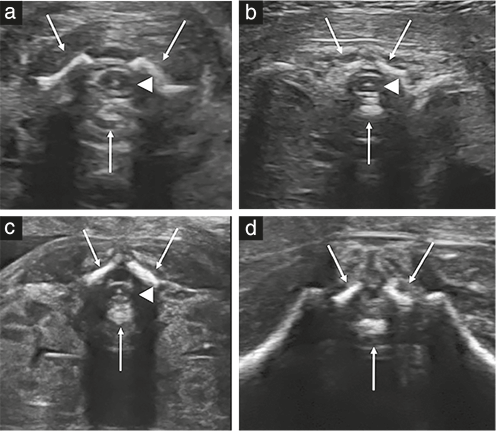
Figure 4 Axial views of fetal spine at different levels: (a) cervical,
(b) thoracic, (c) lumbar and (d) sacral. Arrows indicate three
ossification centers of vertebrae, and arrowheads indicate spinal
cord, which is observed at cervical, thoracic and lumbar levels.
Hyperechogenic dot corresponds to central canal of medulla. At
sacral level (d), only fibers of cauda equina are observed. Note thin
strip of fluid behind cord at all levels and intact skin overlying spine.
fetal weight estimation if the endpoint of the measurement
is to exclude microcephaly.
Measurement of the atrium is recommended because
several studies suggest that this is the most effective
approach for assessing the integrity of the ventricular
system18, and ventriculomegaly is a frequent marker
of abnormal cerebral development. Measurement is
performed at the level of the glomus of the choroid
plexus, perpendicular to the ventricular cavity, positioning
the calipers inside the echoes generated by the lateral
walls (Figure 5). This measurement remains stable in
the second and early third trimesters, with a mean
diameter between 6 and 8mm18,27; it is considered normal
when <10mm27–31. Although this cut-off was determined
several years ago, it remains valid even with more modern
equipment, particularly at midgestation. Therefore, an
atrial width ≥10mm should be considered suspicious. It
is useful to emphasize here that: (1) the atrial width may
change during gestation, either increasing or decreasing,
and (2) moderate asymmetry in atrial width between the
two sides should be considered normal, if both atria
measure <10mm32,33.
The transverse cerebellar diameter increases by about
1mm per week of pregnancy between 14 and 21
gestational weeks. This measurement, along with the HC
and BPD, is helpful to assess fetal growth. In cases in
which the anteroposterior diameter of the cisterna magna
should be measured (because it is subjectively considered
abnormal), the calipers should be positioned in a correct
transcerebellar plane, between the outer edge of the most
dorsal point of the cerebellar vermis and the internal
side of the occipital bone. A normal measurement is
2–10mm34. With dolicocephaly, measurements slightly
larger than 10mm may be encountered.
In a low-risk midtrimester pregnancy, if the transventricular
and transcerebellar planes are obtained
satisfactorily, the head measurements (HC in particular)
are within normal limits for gestational age, the atrial
width is <10mm and the cisterna magna width is
between 2 and 10 mm, many cerebral malformations
are excluded, the risk of a CNS anomaly that can be
diagnosed at this gestational age is exceedingly low and
further examinations are not indicated10.
Screening examination of fetal brain before 18 weeks
Recommendation
If a screening ultrasound examination is carried out
before 18 gestational weeks, efforts should be made
to visualize and document the transventricular and
transcerebellar planes (GOOD PRACTICE POINT).
Fetal ultrasound examinations are being performed
increasingly during the last few weeks of the first
trimester and the early second trimester4,8. These
examinations include evaluation of the brain, but, until
now, there have been no clinical guidelines for its examination.
In our opinion, every fetal brain examination
should include, at the very least, visualization of the
transventricular and transcerebellar planes (Figure 6).
Due to the rapid and dynamic developmental changes
of the brain that occur both during pregnancy and after
delivery, the patient should be informed not only of the
technical limitations of these examinations but also of
those related to temporal issues.
Indications for targeted fetal neurosonography
Recommendation
If suspicion of a brain or spinal abnormality is
raised during the obstetric ultrasound screening
examination, the woman should undergo targeted fetal
neurosonography as a diagnostic examination (GOOD PRACTICE POINT).
Targeted fetal neurosonography is a dedicated, multiplanar,
diagnostic examination for fetuses at high risk or
with suspicion of CNS or spinal malformations. Indications
for referral are shown in Table 2. Analogous
to fetal echocardiography in the context of congenital
heart disease, neurosonography has a much greater diagnostic
potential than does the screening transabdominal
ultrasound examination, and it is particularly helpful in
the evaluation of complex malformations. Of note, this
examination requires a high level of expertise in both
transabdominal and transvaginal approaches as well as in
three-dimensional ultrasound, which is still not available
in many settings worldwide. In addition to the planes
used in the screening examination, it requires coronal
and sagittal views. All details regarding the technical and
practical aspects of targeted fetal neurosonography are
addressed in Part 2 of this Guideline.
Indications for fetal brain MRI
Recommendation
Fetal brain MRI should be indicated by the findings of the expert performing the targeted neurosonographic examination. It is not appropriate to request MRI based only on suspicion of brain abnormality raised at screening ultrasound (GOOD PRACTICE POINT).
Table 2 Indications for targeted fetal neurosonography
- Suspicion of CNS or spinal malformation at routine screening ultrasound
- Suspicion of CNS or spinal malformation at nuchal translucency scan
- Family history of inheritable CNS or spinal malformations
- Previous pregnancy complicated by fetal brain or spinal malformation
- Fetus with congenital heart disease
- Monochorionic twins
- Suspected congenital intrauterine infection
- Exposure to teratogens known to affect neurogenesis
- Chromosomal microarray findings of unknown significance
CNS, central nervous system
The introduction of MRI for evaluation of the fetal brain has provided a new and important diagnostic tool and has boosted research into and education on the complexities of the developing brain35,36. ISUOG Guidelines for the performance and reporting of fetal MRI have been published recently and provide important information on this technique37. However, stricter adherence to standard referral protocols is mandatory in order to avoid requests for fetal brainMRI directly from the operator performing a screening examination or a scan that is marginally more advanced than screening38,39. Inappropriate referrals have resulted in both a falsely high rate of clinically relevant malformations being detected only byMRI (and published as such) and an exponential rise in fetal brain MRI requests for questionable sonographic findings. In fact, when the results of these publications are analyzed carefully, the clinical usefulness of MRI in fetuses with suspicion of a CNS anomaly is much lower40,41. Furthermore, the issue of high rates of false-positive MRI findings has been raised recently42. It is therefore important that fetal brain MRI is performed only after, and to complement, a neurosonographic examination, and only if indicated by an expert.
Guideline authors
This Guideline was produced on behalf of the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) by the following authors, and peer reviewed by the Clinical Standards Committee. G. Malinger, Division of Ultrasound in Obstetrics and Gynecology, Lis Maternity Hospital, Tel Aviv Sourasky Medical Center, Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel D. Paladini, Fetal Medicine and Surgery Unit, Istituto G. Gaslini, Genoa, Italy K. K. Haratz, Division of Ultrasound in Obstetrics and Gynecology, Lis Maternity Hospital, Tel Aviv Sourasky Medical Center, Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel A. Monteagudo, Carnegie Imaging for Women, Obstetrics, Gynecology and Reproductive Science, Icahn School of Medicine at Mount Sinai, New York, NY, USA G. Pilu, Obstetric Unit, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy I. E. Timor-Tritsch, Division of Obstetrical and Gynecological Ultrasound, NYU School of Medicine, New York, NY, USA
Citation
This Guideline should be cited as: ‘MalingerG, PaladiniD, Haratz KK, Monteagudo A, Pilu G, Timor-Tritsch IE. ISUOG Practice Guidelines (updated): sonographic examination of the fetal central nervous system. Part 1: performance of screening examination and indications for targeted neurosonography. Ultrasound Obstet Gynecol 2020; 56: 476–484.
References
1. Myrianthopoulos NC. Epidemiology of central nervous system malformations. In
Handbook of Clinical Neurology,Vinken PJ, Bruyn GW (eds). Elsevier: Amsterdam,
1977; 139–171.
2. Salomon LJ, Alfirevic Z, Berghella V, Bilardo C, Hernandez-Andrade E, Johnsen SL,
Kalache K, Leung KY, Malinger G, Munoz H, Prefumo F, Toi A, Lee W, Committee
ICS. Practice guidelines for performance of the routine mid-trimester fetal ultrasound
scan. Ultrasound Obstet Gynecol 2011; 37: 116–126.
3. Timor-Tritsch IE, Rottem S. Transvaginal Sonography. Elsevier: New York, 1988.
4. Rottem S, Bronshtein M, Thaler I, Brandes JM. First trimester transvaginal
sonographic diagnosis of fetal anomalies. Lancet 1989; 1: 444–445.
5. Johnson SP, Sebire NJ, Snijders RJ, Tunkel S, Nicolaides KH. Ultrasound screening
for anencephaly at 10–14weeks of gestation. Ultrasound Obstet Gynecol 1997; 9:
14–16.
6. Ghi T, Pilu G, Savelli L, Segata M, Bovicelli L. Sonographic diagnosis of congenital
anomalies during the first trimester. Placenta 2003; 24 (Suppl B): S84–87.
7. Monteagudo A, Timor-Tritsch IE. Normal sonographic development of the central
nervous system from the second trimester onwards using 2D, 3D and transvaginal
sonography. Prenat Diagn 2009; 29: 326–339.
8. Chaoui R, Nicolaides KH. Detecting open spina bifida at the 11–13-week scan by
assessing intracranial translucency and the posterior brain region: mid-sagittal or
axial plane? Ultrasound Obstet Gynecol 2011; 38: 609–612.
9. D’Antonio F, Familiari A, Thilaganathan B, Papageorghiou AT, Manzoli L, Khalil A,
Bhide A. Sensitivity of first-trimester ultrasound in the detection of congenital
anomalies in twin pregnancies: population study and systematic review. Acta Obstet
Gynecol Scand 2016; 95: 1359–1367.
10. Filly RA, Cardoza JD, Goldstein RB, Barkovich AJ. Detection of fetal central nervous
system anomalies: a practical level of effort for a routine sonogram. Radiology 1989;
172: 403–408.
11. Falco P, Gabrielli S, Visentin A, Perolo A, Pilu G, Bovicelli L. Transabdominal
sonography of the cavum septum pellucidum in normal fetuses in the second and
third trimesters of pregnancy. Ultrasound Obstet Gynecol 2000; 16: 549–553.
12. Malinger G, Lev D, Oren M, Lerman-Sagie T. Non-visualization of the cavum septi
pellucidi is not synonymous with agenesis of the corpus callosum. Ultrasound Obstet
Gynecol 2012; 40: 165–170.
13. Paladini D, Pastore G, Cavallaro A, Massaro M, Nappi C. Agenesis of the fetal corpus
callosum: sonographic signs change with advancing gestational age. Ultrasound
Obstet Gynecol 2013; 42: 687–690.
14. MalingerG, Lev D, Kidron D, Heredia F,Hershkovitz R, Lerman-Sagie T.Differential
diagnosis in fetuses with absent septum pellucidum. Ultrasound Obstet Gynecol
2005; 25: 42–49.
15. Shen O, Gelot AB, MoutardML, Jouannic JM, Sela HY, Garel C. Abnormal shape of
the cavum septi pellucidi: an indirect sign of partial agenesis of the corpus callosum.
Ultrasound Obstet Gynecol 2015; 46: 595–599.
16. Karl K, Esser T, Heling KS, Chaoui R. Cavum septi pellucidi (CSP) ratio: a marker
for partial agenesis of the fetal corpus callosum. Ultrasound Obstet Gynecol 2017;
50: 336–341.
17. Cardoza JD, Filly RA, Podrasky AE. The dangling choroid plexus: a sonographic
observation of value in excluding ventriculomegaly. AJR Am J Roentgenol 1988;
151: 767–770.
18. Cardoza JD, Goldstein RB, Filly RA. Exclusion of fetal ventriculomegaly with a
single measurement: the width of the lateral ventricular atrium. Radiology 1988;
169: 711–714.
19. Mahony BS, Nyberg DA, Hirsch JH, Petty CN, Hendricks SK, Mack LA. Mild
idiopathic lateral cerebral ventricular dilatation in utero: sonographic evaluation.
Radiology 1988; 169: 715–721.
20. Pilu G, Reece EA, Goldstein I, Hobbins JC, Bovicelli L. Sonographic evaluation of the
normal developmental anatomy of the fetal cerebral ventricles: II. The atria. Obstet
Gynecol 1989; 73: 250–256.
21. Pretorius DH, Kallman CE, Grafe MR, Budorick NE, Stamm ER. Linear echoes in
the fetal cisterna magna. J Ultrasound Med 1992; 11: 125–128.
22. Bromley B, Nadel AS, Pauker S, Estroff JA, Benacerraf BR. Closure of the cerebellar
vermis: evaluation with second trimester US. Radiology 1994; 193: 761–763.
23. Shepard M, Filly RA. A standardized plane for biparietal diameter measurement.
J Ultrasound Med 1982; 1: 145–150.
24. Snijders RJ, Nicolaides KH. Fetal biometry at 14–40 weeks’ gestation. Ultrasound
Obstet Gynecol 1994; 4: 34–48.
25. Bahlmann F, Reinhard I, Schramm T, Geipel A, Gembruch U, von Kaisenberg CS,
Schmitz R, Stupin J, Chaoui R, Karl K, Kalache K, Faschingbauer F, Ponnath M,
Rempen A, Kozlowski P. Cranial and cerebral signs in the diagnosis of spina bifida
between 18 and 22weeks of gestation: a German multicentre study. Prenat Diagn
2015; 35: 228–235.
26. Jian N, Lin N, Tian MM, Zhang S, Li G, Zhao H, Xiao LX, Liang WJ, Lin XT.
Normal development of costal element ossification centers of sacral vertebrae in the
fetal spine: a postmortem magnetic resonance imaging study. Neuroradiology 2019;
61: 183–193.
27. Pilu G, Falco P, Gabrielli S, Perolo A, Sandri F, Bovicelli L. The clinical significance
of fetal isolated cerebral borderline ventriculomegaly: report of 31 cases and review
of the literature. Ultrasound Obstet Gynecol 1999; 14: 320–326.
28. Gaglioti P, Danelon D, Bontempo S, Mombro M, Cardaropoli S, Todros T. Fetal
cerebral ventriculomegaly: outcome in 176 cases. Ultrasound Obstet Gynecol 2005;
25: 372–377.
29. Pagani G, Thilaganathan B, Prefumo F. Neurodevelopmental outcome in isolated
mild fetal ventriculomegaly: systematic review and meta-analysis. Ultrasound Obstet
Gynecol 2014; 44: 254–260.
30. Mehlhorn AJ, Morin CE, Wong-You-Cheong JJ, Contag SA. Mild fetal cerebral
ventriculomegaly: prevalence, characteristics, and utility of ancillary testing in cases
presenting to a tertiary referral center. Prenat Diagn 2017; 37: 647–657.
31. Scelsa B, Rustico M, Righini A, Parazzini C, Balestriero MA, Introvini P, Spaccini L,
Mastrangelo M, Lista G, Zuccotti GV, Veggiotti P. Mild ventriculomegaly from
fetal consultation to neurodevelopmental assessment: A single center experience and
review of the literature. Eur J Paediatr Neurol 2018; 22: 919–928.
32. Atad-Rapoport M, Schweiger A, Lev D, Sadan-Strul S, Malinger G, Lerman-Sagie T.
Neuropsychological follow-up at school age of children with asymmetric ventricles
or unilateral ventriculomegaly identified in utero. BJOG 2015; 122: 932–938.
33. Sadan S, Malinger G, Schweiger A, Lev D, Lerman-Sagie T. Neuropsychological
outcome of children with asymmetric ventricles or unilateral mild ventriculomegaly
identified in utero. BJOG 2007; 114: 596–602.
34. Mahony BS, Callen PW, Filly RA, Hoddick WK. The fetal cisterna magna. Radiology
1984; 153: 773–776.
35. Wimberger-Prayer D. Fetal MRI. Springer-Verlag: Berlin, Heidelberg, 2011.
36. Garel C. MRI of the Fetal Brain. Normal Development and Cerebral Pathologies.
Springer-Verlag: Berlin, Heidelberg, 2004.
37. Prayer D, Malinger G, Brugger PC, Cassady C, De Catte L, De Keersmaecker B,
Fernandes GL, Glanc P, Goncalves LF, Gruber GM, Laifer-Narin S, LeeW,Millischer
AE, Molho M, Neelavalli J, Platt L, Pugash D, Ramaekers P, Salomon LJ, Sanz M,
Timor-Tritsch IE, Tutschek B, Twickler D, Weber M, Ximenes R, Raine-Fenning N.
ISUOG Practice Guidelines: performance of fetal magnetic resonance imaging.
Ultrasound Obstet Gynecol 2017; 49: 671–680.
38. Levine D, Barnes PD, Robertson RR, Wong G, Mehta TS. Fast MR imaging of fetal
central nervous system abnormalities. Radiology 2003; 229: 51–61.
39. Griffiths PD, Bradburn M, Campbell MJ, Cooper CL, Graham R, Jarvis D, Kilby
MD, Mason G, Mooney C, Robson SC, Wailoo A, on behalf of the MERIDIAN
collaborative group. Use ofMRI in the diagnosis of fetal brain abnormalities in utero
(MERIDIAN): a multicentre, prospective cohort study. Lancet 2017; 389: 538–546.
40. Malinger G, Paladini D, Pilu G, Timor-Tritsch IE. Fetal cerebral magnetic resonance
imaging, neurosonography and the brave new world of fetal medicine. Ultrasound
Obstet Gynecol 2017; 50: 679–680.
41. Paladini D, Donarini G, Rossi A. Indications for MRI in fetal isolated mild
ventriculomegaly . . . ‘And then, there were none’. Ultrasound Obstet Gynecol 2019;
54: 151–155.
42. Birnbaum R, Ben-Sira L, Lerman-Sagie T, Malinger G. The use of fetal
neurosonography and brain MRI in cases of cytomegalovirus infection during
pregnancy: A retrospective analysis with outcome correlation. Prenat Diagn 2017;
37: 1335–1342.
APPENDIX 1 Grades of recommendation and levels of evidence used in ISUOG Guidelines
Classification of evidence levels
| 1++ | High-quality meta-analyses, systematic reviews of randomized controlled trials or randomized controlled trials with very low risk of bias |
| 1+ | Well-conducted meta-analyses, systematic reviews of randomized controlled trials or randomized controlled trials with low risk of bias |
| 1- | Meta-analyses, systematic reviews of randomized controlled trials or randomized controlled trials with high risk of bias |
| 2++ | High-quality systematic reviews of case–control or cohort studies or high-quality case–control or cohort studies with very low risk of confounding, bias or chance and high probability that the relationship is causal |
| 2+ | Well-conducted case–control or cohort studies with low risk of confounding, bias or chance and moderate probability that the relationship is causal |
| 2- | Case–control or cohort studies with high risk of confounding, bias or chance and significant risk that the relationship is not causal |
| 3 | Non-analytical studies, e.g. case reports, case series |
| 4 | Expert opinion |
Grades of recommendation
| A | At least one meta-analysis, systematic review or randomized controlled trial rated as 1++ and applicable directly to the target population; or a systematic review of randomized controlled trials or a body of evidence consisting principally of studies rated as 1+ applicable directly to the target population and demonstrating overall consistency of results |
| B | Body of evidence including studies rated as 2++ applicable directly to the target population and demonstrating overall consistency of results; or extrapolated evidence from studies rated as 1++ or 1+ |
| C | Body of evidence including studies rated as 2+ applicable directly to the target population and demonstrating overall consistency of results; or extrapolated evidence from studies rated as 2++ |
| D | Evidence level 3 or 4; or evidence extrapolated from studies rated as 2+ |
| Good practice point | Recommended best practice based on the clinical experience of the guideline development group |
