Probe Care
Probe Care
At GE Healthcare, we know that the probe is the most sensitive element of an ultrasound system.
This is why we have in mind to propose you a specific offering dedicated to your probes.
You will find in this section everything you need to know to take care of your probes, to ensure correct hygiene for your patients and staff and maintenance solutions when needed.

Probe Care Resources
Probe Maintenance Solution
Scientific evidence indicates over 1 in 3 probes in clinical use today have some form of failure, which may put clinical diagnosis at risk1,2. How can you ensure that your ultrasound system is performing as expected?
GE Healthcare facilitates reducing your probe management costs while maximizing continuity of your medical activity through multi-brand coverage of your entire fleet of probes. We help you bring probe care to the next level, partnering with you in proactive management of your probe fleet.
1st probes manufacturer
150 000 probes per year
Certified tests
and processes to stay in manufacturer’s specifications
70 experts
with 5 years experience
4 major repair centers
Zipf (Austria), Freiburg (Germany),
OAK Creek (USA), Horten (Norway)
3 000 diagnosed probes*
1 000 repaired probes per year*
650 loaners available
immediately if in stock
Diagnosis
within 48h
Repair time
between 5 and 10 days depending on failure
Discover our multi-brand probe care solutions
Taking care of your probe is our duty and that's why we created our services portfolio, to best fit all your needs.
Repair & replacement
Enjoy our multi-brand probe repair & replacement offering.
Our team will guide you through the right solution depending on the issue faced.
Fleet health check
A comprehensive health check of your fleet of probes that can
help you reduce the incidence of damaged probes in use, and the possible misdiagnosis and
re-examinations.
Education
Help maximize your probe performance, benefit from educational
resources on the recommended care, handling and maintenance procedures of your probes by refering to
our probe care manual available on our Care Card.
Loaner
We provide you with a loaner while your defective equipment is being
taken care of.
Total care: fleet management
With Total Care solution, GE Healthcare supports you in managing your entire fleet of probes, in a
proactive way. We partner with you to help you run your clinical activity with a high level of
confidence.
Probe Care App
GE Healthcare Probe Care Europe: Quality for you
Probe Care Resources
Probe Hygiene
Everything you need to know about ultrasound probe disinfection
Preventing cross-contamination is an important topic in the healthcare world. When it comes to ultrasound examinations, the question everyone is asking is: “How can I make sure my ultrasound probe is cleaned and ready for the next exam?”
Why is probe disinfection needed?
Probe disinfection is critical in protecting patient and hospital staff:
- There are risks associated with not doing proper probe reprocessing. For example, up to 7% of ultrasound probes were found to be contaminated with human papillomavirus (HPV) after disinfection with low level wipes.1,2,3
- Spaulding Classification is a widely used framework that specifies medical device reprocessing requirements based on the intended use.
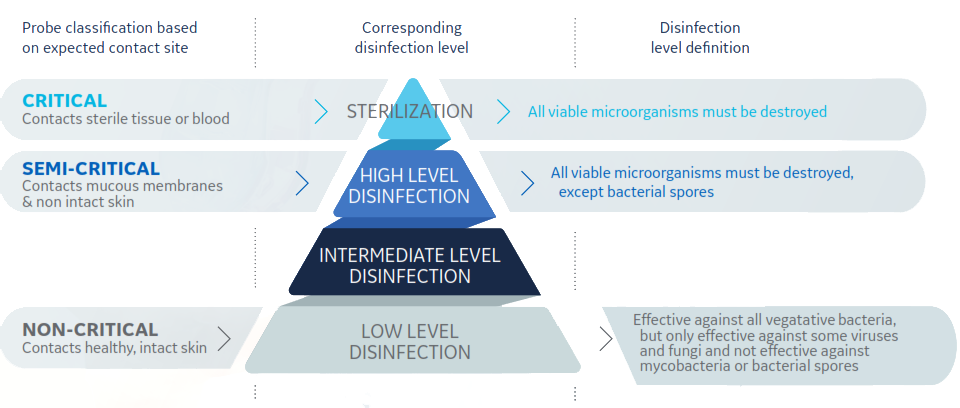
- The probe disinfection landscape in Europe is changing. A 2016 European Society of Radiology (ESR) study found a wide range of practices across Europe, with a need to raise awareness among practitioners of the importance of infection prevention and control measures.
Based on that, the ESR issued a best practice recommendation in November 2017:
- High level disinfection of probes is mandatory for endocavitary ultrasound and all interventions.
- Automated systems offer standardized and reproducible decontamination processes, helping to avoid operator-associated errors or variations.
- Dedicated transducer covers should be used for endocavitary ultrasound and all interventions.
- Sterile gel should be used for endocavitary ultrasound and all interventions.
Some countries have already started to develop their own regulations further:
- In 2017, Ireland and Scotland made high level disinfection mandatory between examinations.4
- In 2019 the French Ministry of Health published data sheets about endocavity probe reprocessing for healthcare professionals.5
- Other countries recommend strongly the same practices.6
- Casalegno JS, Le Bail Carval K, Eibach D, Valdeyron ML, Lamblin G, Jacquemoud H, et al. High risk HPV contamination of endocavity vaginal ultrasound probes: an underestimated route of nosocomial infection? PloS one. 2012;7(10):e48137
- Ma ST, Yeung AC, Chan PK, Graham CA. Transvaginal ultrasound probe contamination by the human papillomavirus in the emergency department. Emergency medicine journal. 2013;30(6):472–5
- M’Zali F, Bounizra C, Leroy S, Mekki Y, Quentin-Noury C, Kann M. Persistence of Microbial Contamination on Transvaginal Ultrasound Probes despite Low-Level Disinfection Procedure. PloS one. 2014;9(4):e93368.
- https://hpspubsrepo.blob.core.windows.net/hps-website/nss/1937/documents/1_RES-183-1-v1.pdf (IIrish HSE Guidance for Decontamination of Semi‐critical Ultrasound Probes QPSD-GL-028-1- 2017) Health Service Executive (HSE) Quality Improvement Division (2017). HSE Guidance for Decontamination of Semi‐critical Ultrasound Probes; Semi‐invasive and Non‐invasive Ultrasound Probes. Document: QPSD-GL-028-1
- https://solidarites-sante.gouv.fr/soins-et-maladies/qualite-des-soins-et-pratiques/securite/article/prevention-des-risques-d-infection-associes-a-l-utilisation-des-sondes-d
- Werkgroep Infectie Preventie (2017). Reiniging, desinfectie en sterilisatie van medische hulpmiddelen voor hergebruik niet-kritisch, semi-kritisch of kritisch gebruik: 56.
Direzione Sanitaria AUSL Pescara (2009). Linee Guida per la “Corretta gestione di Procedure Assistenziali e Igienico-Sanitarie in Setting di Cura Ospedalieri e Territoriali”: 88.
Kommission für Krankenhaushygiene und Infektionsprävention (KRINKO), and Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM), (2012). Anforderungen an die Hygiene bei der Aufbereitung von Medizinprodukten. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz: 66.
Society and College of Radiographers and British Medical Ultrasound Society (2017). «Guidelines For Professional Ultrasound Practice.» 127.
Welsh Health Technical Memorandum (WHTM) (2014). WHTM 01-06 - Decontamination of flexible endoscopes Part C: Operational management, NHS Wales Shared Services Partnership – Specialist Estates Services: 74
How do you disinfect a probe?
Ultrasound probe disinfection can be done in several ways:
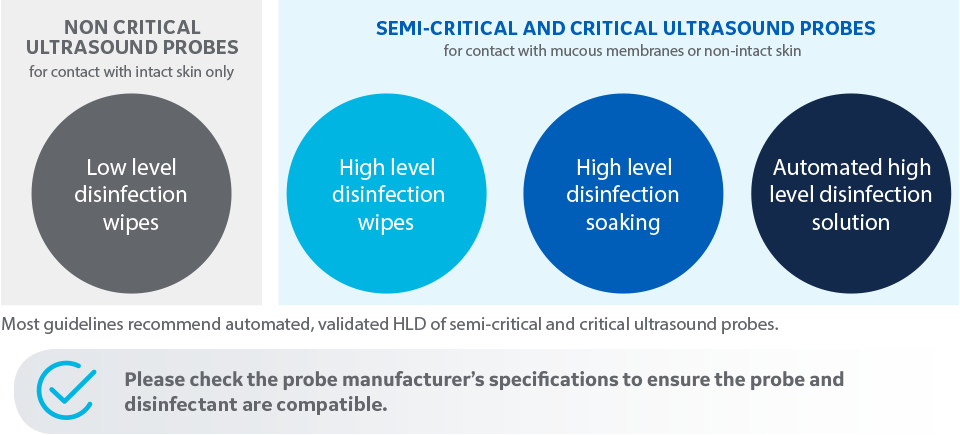
Please check the probe manufacturer’s specifications to ensure the probe and disinfectant are compatible: www.gehealthcare.com/transducers
Learn more about probe disinfection in our e-book.
What does GE recommend?
GE Healthcare recommends in its user manual the following about cleaning and disinfection of ultrasound probes.
“Adequate cleaning and disinfection between patient cases are necessary to prevent disease transmission. All probes must be thoroughly cleaned prior to disinfection. The level of disinfection required is based on patient contact.”
- Probes that contact mucosal or non-intact skin require cleaning followed by high-level disinfection by either soaking or use of an automated system such as TD100®.
- Probes that contact intact skin require cleaning followed by intermediate-level disinfection (wipe or spray).
Transportation & storage
Transport and storage are key when we talk about ultrasound probes.
Probes should be manipulated with care to avoid any failure. If the probe is not
immediately
reused, store the probe in a manner that will protect and keep the probe from being
recontaminated.
To transport effectively and securely TEE probes, you can use TPorter transportation
suitcase from the exam room to the reprocessing room to avoid cross-contamination
and
minimize the risk of probe damage. It helps clearly differentiate soiled probe vs.
disinfected probe.
To securely store disinfected TEE probes, at GE we recommend the following:
TEE probes should be stored in a dry environment, hung vertically and within a HEPA
clean
environment.
You can use the TEE probe storage cabinet.
- It prevents cross-contamination thanks to the separation between the connector and the tip
- It minimizes the potential of airborne contaminants entering the cabinet
- It avoids probe damage thanks to the probe position
- It can store up to 6 probes

UMONIUM38® Neutralis Tissues – high-level disinfection wipes
UMONIUM38® Neutralis Tissues by Laboratoire Huckert’s International are ultrasound disinfection wipes, totally safe for operators, patients, environment and material1, that help you achieve a high-level disinfection2 of surface and endocavitary probes. With these wipes, you can disinfect the entire probe including the handle, the cable and the connector but also the probe environment.
These wipes have a bactericidal, mycobactericidal, fungicidal and sporicidal activity validated under the strictest conditions (closest to operating conditions) and comply with all European standards3.
UMONIUM38® Neutralis Tissues have an excellent performance before disinfection and proven effectiveness against biofilms formation.
They are fully adapted for sensitive environments such as AR and IVF establishments, since they are safe up to embryo cells level and gametes4. It is a product adapted to gynecology environment.
Thanks to a neutral PH (PH = 7), UMONIUM38® Neutralis Tissues are non-corrosive and have multi-material compatibility.
They are an economical and ready-to-use solution. The reprocessing workflow is simple, two wipes are required: one for cleaning and one for disinfection. Each wipe can treat 3 m2 of surface. The shelf life is 3 years after opening the box.
It is an eco-friendly product and the wipes are made of wood-pulp.
How to reprocess your probe with UMONIUM38® Neutralis Tissues?

- EN16615, EN14561, EN14562, EN14563, EN13727, EN13624, EN14476, EN14348
- Achieved by following the recommended reprocessing workflow
- Bactericidal, Mycobactericidal, Fungicidal, Sporicidal validated in 5 seconds according to the American Standard ASTM2967-15
- Tests Mouse Embryo Assay (MEA) and Human Sperm Survival Assay (HSSA)

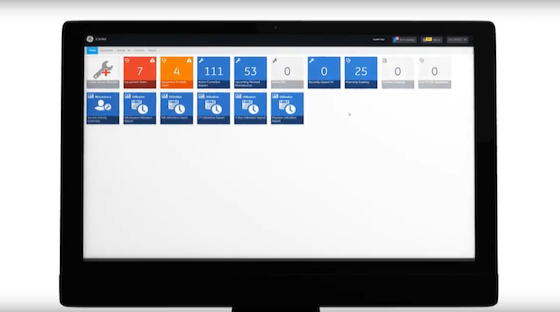
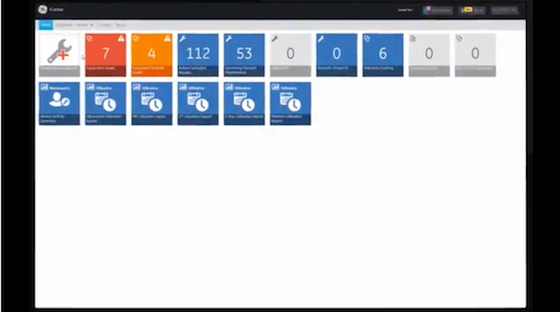
iCenter
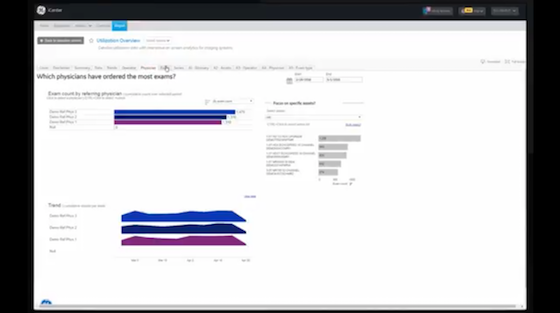
Visualize your data to help optimize assets.
iCenter™ provides insights for hospital managers to improve operational performance, asset utilization, and asset performance.
Fast Facts

Asset Status

Notifications

24/7 Service Request
Better decisions start with better data.
iCenter is a secure, cloud-based asset maintenance and management software tool with the power to simplify the equipment monitoring process for a more efficient workflow and improved healthcare outcomes.
Discover how iCenter works

Start improving your operational performance today.
Join nowUpdateMe mobile app
Get greater insights that help you manage the performance and maintenance of your assets at your fingertips

A real-time application that gives you 24/7 updates and insightful information of your asset status and operations.
With UpdateMe, information is delivered to your Mobile devices 24/7, helping you to make the right decision on time with best insights, an easy way to connect with us, and improve the confidence in delivering patient care and workflow optimization.
Powered by iCenter: iCenter is a secure (cloud-based)** online tool that provides visibility to asset operational and asset utilization data.
Fast facts
Asset status
View up-to-date status of each asset and service history—planned and corrective maintenance, and contract status.
Notifications
Follow asset status and service operations at a glance and receive alerts on critical units you select.
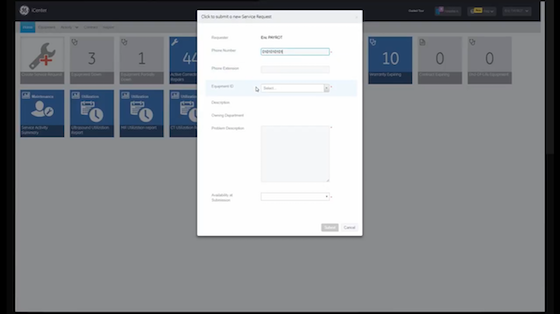
24/7 Service Request
Receive notifications and create a service request anytime, anywhere.
One account, easy access anytime, anywhere
To get the most of your account, download our real time mobile app: UpdateMe.
Asset Management Resources
** UpdateMe is not a medical device
Remote Support
Now more than ever, medicine needs technology

With GE you have now the possibility to access to GE clinical and technical experts during the lifecycle of your system. Having your system connected will benefit you in several ways:
Help save time:
- By minimizing downtime with fast access to GE experts
- 30% of issues on an ultrasound system are repaired remotely and often 3 times quicker*
- If your ultrasound cannot be repaired remotely, 90% of issues are resolved on the 1st visit*
Make full use of your console’s capabilities with clinical support
Keep your ultrasound system up to date:
- With the latest break through technologies thanks to remote software upgrades
- With the latest security features thanks to OS patches installed remotely
Optimize your ultrasound fleet
- Maximize asset utilization and budget management
- Keep an eye on equipment maintenance with iCenter™
The privacy and security of your practice and your patients is controlled We follow GDPR law and we are ISO 27001 certified
To benefit from these services, your system must be connected. Now if you own one of these systems:
- Voluson E10 acquired between September 2017 to September 2018
- Voluson E8 acquired between September 2017 to September 2018
You have the possibility to connect your system by yourself! Please, follow these two steps:
Click here to get access to these videos also in Spanish, Polish, Geman, French
If you face any problem to connect, feel free to contact us.
If you don’t have one of the above system and would like to connect, please contact us:

 Fleet Healthcheck Brochure
Fleet Healthcheck Brochure


 Care Card
Care Card





 iCenter Brochure
iCenter Brochure
 UpdateMe Brochure
UpdateMe Brochure